
儿童和青少年原发性自发性气胸:系统评价
1813年,Laennec首次将胸膜腔内空气聚集导致部分或全部肺不张定义为“气胸”,但是,现代原发性自发性气胸(primary spontaneous pneumothorax,PSP)的特征是由Kjaergaard于1932年第1次提出,即在没有肺部疾病的情况下发生的各种气胸[1]。尽管自发性气胸在儿童健康人群中很常见,但自发性气胸的指南多聚焦于成年人[2-3]。近年来PSP的临床诊治情况没有改变,并仍然依赖经验。由于缺乏儿童和青少年PSP患者管理的证据,导致医生对该疾病的诊治方案不同。本研究的目的是回顾近30年来知识,以总结儿童和青少年PSP的最佳治疗方法。
1 搜索策略和研究选择
我们的搜索策略遵循PRISMA指南[4]。笔者独立收集了Embase和Medline数据库中1988年至2018年的30年间以英文发表的儿科PSP患者的相关文章。初步独立搜索具有以下查询:('primary spontaneous pneumothorax'/exp OR 'primary spontaneous pneumothorax' OR (primary AND spontaneous AND ('pneumothorax'/exp OR pneumothorax))) AND ([school]/ lim OR[adolescent]/lim OR[young adult]/lim) AND[english]/lim AND[1988-2018]/py, ('paediatric primary spontaneous pneumothorax' OR (('paediatric'/exp OR paediatric) AND primary AND spontaneous AND ('pneumothorax'/exp OR pneumothorax)), ('pediatric primary spontaneous pneumothorax' OR (('pediatric'/ exp OR pediatric) AND primary AND spontaneous AND ('pneumothorax'/exp OR pneumothorax)),确定了398篇可能相关的出版物,134篇文章被标题排除,因为重复或与主题无关。其余的审查了摘要,因为没有摘要排除了71篇论文。在剩余的文章中,排除了57篇没有全文和大会报告的文章,其中 5 篇没有摘要。最后,131篇全文文章被纳入本分析。选择过程如图1所示。另有文章和最新指南是由作者从检索文章的参考文献列表中选择的。
2 流行病学
目前尚无儿童PSP的确切发病率报告,但一些作者[5]认为发病率为3.4/100 000,一般人群中,发病率范围为每年每6~18/100 000,并且近几年呈上升趋势(从2001年的5.05/100 000到2013年的7.18/100 000)[6]。该疾病第1个发病高峰在青少年。一项针对19 562名11~40岁患者的大型研究[6]分析了PSP的年龄分层发病率,结果显示:PSP在14岁以后发病率较高,18岁以后逐渐下降,发病高峰在15岁(>20/100 000),25岁下降到稳定水平。因此,根据这些数据,英格兰(原发性和继发性)气胸的流行病学研究[7]发现:急诊科(emergency department,ED)入院的总发病率为每年11.1/100 000,双高峰的2个年龄组为15~34岁和55岁以上,分别与原发性和继发性气胸的发病率相同;与其他研究[5-9]相同,在性别上以男性为主。但Poenaru等[10-11]报道9岁以下儿童在性别方面没有差异。青少年的复发率(31%)高于成人(22岁后<17%)。在10岁以下的儿童人群中,少数研究[12-13]报告了PSP的发生率为0%~15%,相应的在这个年龄段大泡和肺大疱的发生率较低(28%)。这表明在儿童时期,潜在的先天性异常可能是一个诱发因素。PSP患儿普遍具有虚弱体质和更瘦、更高的外胚型体型,这导致几位研究人员将青少年的体重指数(body mass index,BMI)和胸部发育作为危险因素进行调查[14-16],发现PSP患者的BMI低于对照组的平均值(少20 kg/m2)。
一项针对台湾青少年PSP的大型研究[6]表明:青春期前胸廓垂直尺寸的增长导致肺外周实质薄弱,肺泡在负压较高的区域(如心尖)过度扩张,并形成肺气肿样病变,如大泡和肺大疱。
3 病理生理学
PSP的发生可能是多因素的,其确切病因仍有待完全阐明。流行的理论是内脏胸膜下大泡和肺大疱的破裂以及随后空气从肺泡进入胸膜腔[17-18]。肺大疱的发病机制与多种因素有关,包括机体和环境决定因素。在对手术标本的组织病理学分析中,气胸相关成纤维细胞病变(PAFL)被定义为位于外周胸膜上的纤维化,典型的与楔形隔膜和内脏面上弥漫的成纤维细胞灶相关。但PAFL仅在50%的20岁以下患者中发现,其在继发性气胸中不存在。PAFL可能是PSP的一个亚型[19],但目前并不确定PAFL是先前愈合过程的结果还是基于结缔组织疾病的肺气肿样改变(ELC)的表现[20]。有学者[21-23]观察到结缔组织中弹性纤维的破坏是异常的关键,并进行了弹性分解与氧化途径失衡之间的关系的研究。烟草、大麻烟雾以及所有对远端气道有直接炎症作用的化学物质均已被证实是肺损伤和上皮损伤的诱发因素,在肺大疱性等ELC的形成中起重要作用[10,24-25]。有学者[26]分析了空气污染物对因PSP入急诊的影响,发现随着O3、NO2、PM10和PM2.5浓度增加,PSP的发病率分别增加了约15、16、3和5倍。PSP发病的季节性在春季和夏季较高,可以用气压和温度方面的大气变化来解释,但天气决定因素的确切影响尚不清楚[27-30]。
4 诊断
在休息或轻度体力活动期间,若患者出现急性胸部刺伤样的疼痛,须考虑PSP的发作。胸痛是最常见的症状,见于87%的患者,43%的患者出现呼吸困难,5%的患者出现咳嗽[31]。患者症状轻重与肺萎陷程度无关,体格检查不能明确较小的气胸。因为这时气胸常见的体征并不明显,例如呼吸音不对称、在胸部叩诊过清音和患侧的胸壁运动异常。PSP 的诊断必须通过胸部立位X线(chest X-ray,CXR)来确认肺萎陷的边缘。目前的文献数据表明超声检查(ultrasound,US)在儿科急诊PSP中的应用越来越多。与CXR相比,超声检查具有更高的敏感性和相似的特异性。US的汇总敏感性和特异性分别为0.88和 0.99,CXR的汇总敏感性和特异性分别为0.52和1.00。此外,由于它的便携性和没有电离辐射,一些作者指出它特别适用于儿童和青少年;另一方面,使用超声作为诊断PSP的工具受到限制,因为其准确性在很大程度上取决于操作者的技能[32-34]。CT对PSP首次发作的作用存在争议,通常不作为一线诊断工具。由于存在辐射暴露风险,儿科医生和小儿外科医生不推荐进行CT扫描。经计算,这种暴露大约是传统CXR有效剂量的68倍[35]。胸部CT扫描用于检测肺尖部大疱和肺发育不良部位,这些区域被认为是PSP中漏气的原因,亦是复发的危险因素[35-38]。据报道,常规胸部CT扫描(5 mm切片厚度)检测实质泡的特异性为36%~63%,但薄切片高分辨率CT(切片厚度为1 mm的HRCT)提供显著更高的灵敏度,为94%~97%[39-40]。目前仍然缺乏证据表明肺尖部的大泡和肺大疱是PSP复发的高危风险,因此,也没有证据支持PSP患儿进行CT扫描[41]。
5 管理
治疗气胸的首要目的是让塌陷的肺复张。实现这一目标的方法是减轻胸膜腔内的压力,排出空气,以避免张力性气胸及其可能导致的血流动力学改变。治疗气胸的第2个目标可能是防止复发。最佳治疗的选择取决于肺不张的严重程度、漏气的持续性和患者的临床表现。目前用于计算气胸大小的几种方法均有争议。最常用的是Light index、Collins和Rhea方法,它们都基于直立CXR上的估计体积[10]。因此,根据美国胸科医师学会(ACCP)指南,当胸膜顶和肺尖之间的距离>3 cm时,气胸被定义为“大”气胸;或者,根据英国胸科学会(BTS)建议,胸腔边缘的到胸壁>2 cm[2-3,42]。确定气胸的体积后,2种治疗方法被广泛接受:保守治疗和手术治疗。保守治疗包括吸氧观察和针头、猪尾等导管抽吸胸腔内空气。患者在PSP首次发作时或如果临床稳定且无症状,有少量非张力性气胸(Light指数<20%),适合保守治疗。第2种方法是手术治疗,可以通过胸膜切除术、机械或化学胸膜固定术进行。适用于复发或持续漏气非侵入性治疗后肺复张失败的患者[43]。手术治疗的最佳时机仍然存在争议[5,44]。一些观点认为超过50%的患者仅用胸腔导管治疗效果不理想,54%的保守治疗病例4年内PSP复发需要手术治疗。将手术作为首选治疗的提议得到了Chambers等[35,45-50]的支持,应用视频胸腔镜能获得更好的术后疼痛控制和更短的住院时间,比保守治疗节省了42%的成本。另一方面,保守管理也取得了很大进展,许多方案提出了有效的门诊管理方法[42,51-52]。小口径胸腔引流管连接到单向装置(Heimlich阀、Tru-close胸腔通气口),CXR显示引流管正确放置位置。在没有漏气的情况下实现肺的完全复张后,用缝合线和敷料固定胸腔引流管,以防止打结,患者可离院。每日CXR动态对比作为随访评估计划。平均引流时间为3.4±2.5 d。在最初使用小引流管和Heimlich阀从ED出院的50名患者中,有35名从未入院并通过门诊方案进行全面管理[52]。表1列出了本研究中首选保守治疗的文章及其结果。
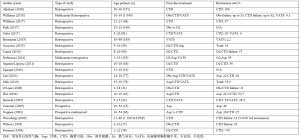
Full table
5.1 胸腔穿刺抽气
正如目前的指南(ACCP、BTS)所推荐的,儿科PSP的一线治疗必须侵入性小。应用非重复式呼吸面罩或鼻插管持续吸氧(2~4 L/min)。目的是通过跨胸膜梯度降低肺泡侧的氮分压,导致氮扩散到肺泡中并逐渐吸收气胸。如果轻度气胸的体积Light指数为20%~40%,则使用胸腔穿刺抽气。针式胸腔穿刺术是将16号针插入穿过锁骨中线的第2肋间水平[53-56]进行简单的手动抽气。直径为8-Fr的猪尾导管可以通过经皮穿刺法(Seldinger)操作放置以重复抽气。用注射器手动抽出空气直到感觉到阻力。复查CXR完全肺复张。如果抽出气量达到4 000 mL而没有肺复张,则必须放置引流管。据报道,胸腔穿刺抽气肺复张成功率可达59.3%,但11%的患者在1周后需要放置引流管,第1年达到26%[56]。
5.2 胸腔引流
胸腔引流置管在锁骨中线上第2肋间或腋中线上第4或第5肋间。大口径引流管的尺寸为16~24 Fr,小口径或猪尾导管尺寸为8~12 Fr。引流管的最佳尺寸一直存在争议[56-60]。Dull等[59]于2002年发表了第1项针对儿童人群的研究,该研究证明了小口径引流管与大口径引流管相比的功效,结果显示:前者有利于疼痛控制,但两组的住院时间(length of stay,LOS)没有显著差异。Kuo等[57]分析了41名18岁以下青少年,接受保守治疗的成功率超过50%,但这与引流管的口径无关。LOS和复发率在使用小口径和大口径引流管治疗的患者之间是相同的。小口径引流管的优势为切口小,从而导致的疼痛小且更加美观。没有证据表明抽气在引流中的作用。在没有抽气仅使用胸管引流的患者中,到第3天,高达70%的患者实现了肺复张。一些学者[10,57,61]认为:当存在持续漏气时,只有少数患者抽气有效,因此,这些病例需要更多侵入性操作。
5.3 手术治疗
由于持续漏气或气胸复发导致不能完全的肺复张时,保守治疗失败,需要手术治疗。手术的最佳时机仍有争议[44-49]。持续漏气的定义因人而异,范围为3~10 d,进行手术的决定也各不相同。这意味着放或不放置引流管的患者的住院时间延长[10]。VATS是首选的治疗方法,因为自开始应用以来,其降低了发病率,实现了更好的疼痛控制,缩短了住院时间,并降低了复发率[62-69]。手术治疗的目标包括切除肺组织上存在的大泡、肺大疱和纤维化区域,许多学者认为这是导致气胸的漏气源,并通过促进胸膜表面间粘连进行胸膜固定术,以避免在气体聚积从而导致的。在过去的几十年里,不同的VATS方法已被开发:从最初的两孔或三孔胸腔镜,到最新的单孔胸腔镜手术((single incision thoracoscopic surgery,SITS)技术,尤其在儿科领域取得了很大的进展[70-76]。SITS是通过穿过腋中线的第7肋间隙中的5~20 mm的单个切口进行的。所有器械都通过同一通路引入胸膜腔,建议有经验的外科医生使用VATS,以免导致胸膜腔运动障碍。引入30°摄像头后,在肺气肿病变更常见的肺上叶尖部,通常通过手术吻合器进行肺大疱楔形切除术[77-79]。此外,即使没有明显的气泡,通常也会在最可疑的区域进行顶端吻合。由学者[80]建议应系统地进行尖段切除,因为该水平的肺大疱可能是VATS后PSP复发的潜在风险。作为内吻合器切除术替代方法,使用带有激光装置的圈套器[81](MBB 100 瓦,德国)[82]与LigaSure血管闭合系统(LVSS)[83](Valleylab,Boulder,Co.,USA)和射频设备等[84]。有学者[85-86]提出用可吸收材料订线覆盖作为附加程序。事实上,儿童和青少年肺大疱的形成是一个动态过程,由脏层胸膜的薄弱和结缔组织的弹力降低引起,后者又被实质切除后缝合周围产生的张力进一步削弱。同时有研究[18,87]在再次手术的术中发现新的肺大疱会从缝钉处出现。并有研究[85-86]提出在缝钉处覆盖以防止在脆弱的肺组织上形成新的肺大疱。肺泡切除后,必须进行胸膜固定术以保持肺与胸壁的黏附,以防止气胸复发肺不张。胸膜固定术的第1种技术是完整的壁层胸膜切除术,由于出血和肋间神经损伤的风险增加,不推荐年轻患者使用。此外,胸膜切除术后坚韧的胸膜融合可能增加了再次胸腔介入手术的困难。目前,实用的技术是机械和化学胸膜固定术[70,88]。机械胸膜固定术是通过VATS进行胸膜表面的磨损,使用无菌材料,如纱布、棉签或刮刀[89-91]。亦有研究[92]使用了电动旋转刷。当壁层胸膜出现出血点和轻度出血时,则治疗有效。胸膜病变也可以通过电灼或氩气束进行,以降低研磨技术可能发生的过度出血的风险[93]。化学胸膜固定术由具有刺激性和硬化剂特性的各种药剂进行——最常见的是滑石粉、碘聚维酮、硝酸银和米诺环素[94-95]。米诺环素已应用于临床,因为低成本和可溶解,可通过细导管给药,便于门诊管理[95]。另一方面,在米诺环素用于胸膜固定术后胸痛更剧烈[96]。Lillegard等[97-99]推荐在化学胸膜固定术中使用自体血液贴片,尤其在儿童和青少年中,其有效、安全地解决了持续漏气问题。最近的综述和系统分析[70,88,96,100]显示:儿科和总体人群中,肺大疱切除术相关的机械和化学胸膜固定术在术后并发症的发生率、结果和LOS方面似乎相同。
6 结果
据报道,PSP如果不进行手术治疗,第1次发作后的复发率为在16%~52%,第2次上升至60%,第3次后上升至80%;在初次发作后2年内最大复发率>60%,对侧肺气胸的风险将从6%上升至18%[36,38,100-101]。Choi等[101]在114名18岁以下的年轻患者中回顾性研究了PSP复发的危险因素,结果显示:与成人患者相比,保守治疗后儿童和青少年的复发率似乎更高(50% vs 30%),但他们没有发现不同治疗类型之间的差异,无论是补充氧气还是放置胸腔引流管;此外,他们发现主要的风险因素是气泡和肺大疱,这与其他研究一致。据报道,在儿童人群HRCT扫描发现的气泡/肺大疱发生率为30.8%~100%,保守治疗后的复发率(50%~100%)被认为与这些“含气病变”有关[102-103]。标准CT扫描确定存在气泡/大泡的情况下报告的复发率为48%,而不存在时为20%。然而,一些研究[12,45,70]却发现CT正常的患者与有肺大疱的患者具有同样高的复发率,其研究结果显示:接受VATS治疗的患者复发率为13%,而仅接受胸腔引流治疗的患者平均复发率为42%;此外,当发现双侧肺大疱时,接受对侧VATS手术的总体风险为28%;当需要手术治疗时,选择的方法是VATS肺大疱切除术和胸膜固定术,因为术后发病率低,并且与保守手术相比,复发风险低且LOS相同。该文献中LOS范围为VATS后2~11 d,平均持续时间为4 d,类似于仅接受胸腔引流治疗的患者。
接受VATS肺大疱切除术和滑石粉胸膜固定术的患者术后12个月和术后5年的肺功能显示FEV1>80%,且DLCO未改变[94,104-105]。Bialas等[70]在包含41名患者的研究中发现VATS肺大疱切除术和机械或化学胸膜固定术后PSP的复发率为5%。一项长期随访96个月的研究记录了VATS手术后的复发率,与其他研究的结果相同(0%~5%),结果表明不同的胸膜固定术不会影响复发率。Shaikhrezai等[49,106-107]在一项包含596名患者研究中,总共进行了644次VATS手术,随访10年,发现在没有进一步气胸发作的情况下,擦伤、取物和胸膜切除术的成功率分别为96.4%、98.9%、97.5%。VATS手术后复发的原因是当看不到气泡时未能识别漏气的来源,以及胸膜固定术不完整,特别是与套管针插入部位。据报道,尖段切除术将复发率从27%降低到3%,为防止术后延迟漏气,一些研究[17]建议即使没有明显的肺大疱,也可进行肺尖切除术。看不见的气泡可能是术后延迟漏气和导致的气胸复发的危险因素,建议切除下叶上段,12.5%行VATS的患者在手术期间可以见到肺大疱[70,80]。当需要再次手术时,先前胸膜固定术后形成的粘连会使重做VATS更加困难,必须考虑转换为开胸手术。Doddoli等[87,108-109]在39名患者的小型研究中发现:重做VATS在近70%的病例中是可行的,开胸手术的转换率为39%,且仅取决于先前胸膜固定术后的粘连程度,而2次手术间隔时间长不影响重复VATS的可行性。
7 讨论
儿科PSP的最佳治疗方案尚未达成一致。目前,基于成人患者的指南因缺乏证据,以及在几个管理问题上缺乏一致性而受到质疑[37]。事实上,诊断和手术技术的进步并没有带来PSP管理的进展。
在传统的定义中,PSP曾被标记为特发性,因为它发生在没有病理情况下的健康的肺中[110]。在切除的肺大疱病理学方面,有学者[19-23]研究了弹性生成的机制并确定了细胞外蛋白质的异常,这表明结缔组织疾病与25岁以下患者的PSP发病率之间存在直接相关性。这些异常可以是遗传性的(马凡氏综合征[13,20,111-112]、Birt-Hogg-Dubè综合征[113])或后天性的(厌食症[114]、较低的BMI[115]、吸烟习惯[7]),但未将其作为PSP复发风险分层的诱发因素,而且这些年来治疗选择没有发生实质性变化。所有患者的手术指征仍然如下[10]:1)第2次同侧气胸;2)第1次对侧气胸;3)双侧气胸;4)持续漏气;5)自发性血胸;6)有气压伤风险的职业(或拟从事的职业)。但是,近年来的研究在筛选PSP复发风险较高的人群时将手术治疗作为一线方案。一些作者提议现在应该重新考虑PSP的管理[110]。VATS自1990年问世后,现已成为首选的手术方法[62]。在儿科患者中,VATS在术后并发症、术后疼痛控制、LOS和复发率方面效果更好。一项比较VATS和开放手术的成本和收益分析[68-69]发现VATS具有显著优势。这些数据支持在PSP首次发作时选择VATS,但由于缺乏证据支持这一理论,外科医生尚未达成共识[35,45-50]。此外,随着近年来的进一步发展,SITS得到了广泛的应用[70-76]。这种技术有望改善术后疼痛控制和整体效果,但尚无前瞻性研究对VATS和SITS进行比较。当复发风险与可识别的肺部疾病无关时,国际指南仍推荐将保守方法作为儿童和青少年的一线治疗,但目前的方案已有明显的改变[2,3,10-13],其建议3种非手术方法:1)针吸;2)猪尾小口径导管;3)大口径导管胸廓造口术。即使这些方法在成年人是常规程序,耐受性是儿科需要考虑的一个方面,因为差异大、置管常常需要镇静、甚至全麻。手动胸腔穿刺抽气的效果有争议,常推荐首选放置猪尾管[116]。
一般来说,这些保守的手术需要院内监测,时间取决于医生的习惯。最近推荐的一种将小口径引流管连接到Heimlich瓣膜的门诊方案,有效且并发症少。对18项使用Heimlich瓣膜进行门诊动态管理的研究的系统评价报告[117]称:总体成功率为86%、78%的病例管理顺利。由于PSP复发的风险较高,未来院内管理将有针对性地选择考虑采用微创方法进行早期手术的患者。对于没有易感因素的患者,如果可能,也应建议在儿童和青少年中使用连接单向阀的小型胸腔引流管进行门诊管理[110,118]。总之,儿科患者的PSP需要肺病学家、儿内科医生、儿外科和胸外科医生的参与,这可能是关于疾病治疗的共同策略存在不同观点的原因之一。根据多学科专家组声明,直到今天,该病的病理学研究仍不足,还需要进行进一步研究,以提供更有力的证据用于制定该病的方案。
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Paolo Scanagatta) for the series “Pediatric Thoracic Surgery” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm.2019.04.01/coif). The series “Pediatric Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Papagiannis A, Lazaridis G, Zarogoulidis K, et al. Pneumothorax: an up to date "introduction". Ann Transl Med 2015;3:53. [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [Crossref] [PubMed]
- Henry M, Arnold T, Harvey J, et al. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58:ii39-52. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med 2009;6:e1000100 [Crossref] [PubMed]
- Lopez ME, Fallon SC, Lee TC, et al. Management of the pediatric spontaneous pneumothorax: is primary surgery the treatment of choice? Am J Surg 2014;208:571-6. [Crossref] [PubMed]
- Huang YH, Chang PY, Wong KS, et al. An Age-Stratified Longitudinal Study of Primary Spontaneous Pneumothorax. J Adolesc Health 2017;61:527-32. [Crossref] [PubMed]
- Gupta D, Hansell A, Nichols T, et al. Epidemiology of pneumothorax in England. Thorax 2000;55:666-71. [Crossref] [PubMed]
- Mitani A, Hakamata Y, Hosoi M, et al. The incidence and risk factors of asymptomatic primary spontaneous pneumothorax detected during health check-ups. BMC Pulm Med 2017;17:177. [Crossref] [PubMed]
- Fujino S, Inoue S, Tezuka N, et al. Physical development of surgically treated patients with primary spontaneous pneumothorax. Chest 1999;116:899-902. [Crossref] [PubMed]
- Robinson PD, Cooper P, Ranganathan SC. Evidence-based management of paediatric primary spontaneous pneumothorax. Paediatr Respir Rev 2009;10:110-7; quiz 117. [Crossref] [PubMed]
- Poenaru D, Yazbeck S, Murphy S. Primary spontaneous pneumothorax in children. J Pediatr Surg 1994;29:1183-5. [Crossref] [PubMed]
- Seguier-Lipszyc E, Elizur A, Klin B, et al. Management of primary spontaneous pneumothorax in children. Clin Pediatr (Phila) 2011;50:797-802. [Crossref] [PubMed]
- Kaslow J, Bickel S, Wiesenauer C, et al. Pediatric Spontaneous Pneumothorax: Our Experience and a Review of the Literature. Pediatr Allergy Immunol Pulmonol 2018;31:208-14. [Crossref]
- Chang PY, Wong KS, Lai JY, et al. Rapid increase in the height and width of the upper chest in adolescents with primary spontaneous pneumothorax. Pediatr Neonatol 2015;56:53-7. [Crossref] [PubMed]
- Ayed AK, Bazerbashi S, Ben-Nakhi M, et al. Risk factors of spontaneous pneumothorax in Kuwait. Med Princ Pract 2006;15:338-42. [Crossref] [PubMed]
- Akkas Y, Peri NG, Kocer B, et al. A novel structural risk index for primary spontaneous pneumothorax: Ankara Numune Risk Index. Asian J Surg 2017;40:249-53. [Crossref] [PubMed]
- Ayed AK, Chandrasekaran C, Sukumar M. Video-assisted thoracoscopic surgery for primary spontaneous pneumothorax: clinicopathological correlation. Eur J Cardiothorac Surg 2006;29:221-5. [Crossref] [PubMed]
- Ota H, Kawai H, Kuriyama S. The Presence of a Reticulated Trabecula-Like Structure Increases the Risk for the Recurrence of Primary Spontaneous Pneumothorax after Thoracoscopic Bullectomy. Ann Thorac Cardiovasc Surg 2016;22:139-45. [Crossref] [PubMed]
- Belchis DA, Shekitka K, Gocke CD. A unique, histopathologic lesion in a subset of patients with spontaneous pneumothorax. Arch Pathol Lab Med 2012;136:1522-7. [Crossref] [PubMed]
- Sauter JL, Butnor KJ. Pathological findings in spontaneous pneumothorax spec-imens: does the incidence of unexpected clinically significant findings justify routine histological examination? Histopathology 2015;66:675-84. [Crossref] [PubMed]
- Chen YW, Chiu WC, Chou SH, et al. High Nrf2 expression in alveolar type I pneumocytes is associated with low recurrences in primary spontaneous pneu-mothorax. Kaohsiung J Med Sci 2017;33:496-502. [Crossref] [PubMed]
- Chiu CY, Chen TP, Chen JR, et al. Overexpression of matrix metalloproteinase-9 in adolescents with primary spontaneous pneumothorax for surgical intervention. J Thorac Cardiovasc Surg 2018;156:2328-2336.e2. [Crossref] [PubMed]
- Hirai Y, Muragaki Y, Itoh S, et al. Fibulin-5 protein is reduced in the lung of patients with spontaneous pneumothorax who are under 25 years old. Ann Thorac Cardiovasc Surg 2012;18:200-5. [Crossref] [PubMed]
- Bintcliffe O, Maskell N. Spontaneous pneumothorax. BMJ 2014;348:g2928. [Crossref] [PubMed]
- Fiorelli A, Accardo M, Vicidomini G, et al. Does cannabis smoking predispose to lung bulla formation? Asian Cardiovasc Thorac Ann 2014;22:65-71. [Crossref] [PubMed]
- Park JH, Lee SH, Yun SJ, et al. Air pollutants and atmospheric pressure increased risk of ED visit for spontaneous pneumothorax. Am J Emerg Med 2018;36:2249-53. [Crossref] [PubMed]
- Motono N, Maeda S, Honda R, et al. Atmospheric temperature and pressure in-fluence the onset of spontaneous pneumothorax. Clin Respir J 2018;12:557-62. [Crossref] [PubMed]
- Oruç M, Şahin A, Dursun R, et al. Do Meteorological Changes Have an Effect on The Occurence of Spontaneous Pneumothorax? Turk Thorac J 2016;17:89-92. [Crossref] [PubMed]
- Haga T, Kurihara M, Kataoka H, et al. Influence of weather conditions on the onset of primary spontaneous pneumothorax: positive association with decreased atmospheric pressure. Ann Thorac Cardiovasc Surg 2013;19:212-5. [Crossref] [PubMed]
- Yamac ME, Karapolat S, Turkyilmaz A, et al. Relationship of spontaneous pneumothorax cases seen in Eastern Black Sea region with meteorological changes. Int J Biometeorol 2017;61:1493-8. [Crossref] [PubMed]
- Robinson PD, Blackburn C, Babl FE, et al. Paediatric Emergency Departments International Collaborative (PREDICT) research network. Management of paediatric spontaneous pneumothorax: a multicentre retrospective case series. Arch Dis Child 2015;100:918-23. [Crossref] [PubMed]
- Ding W, Shen Y, Yang J, et al. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest 2011;140:859-66. [Crossref] [PubMed]
- Unlüer EE, Karagöz A. Bedside ultrasonographic diagnosis of pneumothorax. Interv Med Appl Sci 2014;6:133-6. [Crossref] [PubMed]
- Ng C, Tsung JW. Point-of-care ultrasound for assisting in needle aspiration of spontaneous pneumothorax in the pediatric ED: a case series. Am J Emerg Med 2014;32:488.e3-8. [Crossref] [PubMed]
- Soler LM, Raymond SL, Larson SD, et al. Initial primary spontaneous pneumothorax in children and adolescents: Operate or wait? J Pediatr Surg 2018;53:1960-3. [Crossref] [PubMed]
- Guimaraes CV, Donnelly LF, Warner BW. CT findings for blebs and bullae in children with spontaneous pneumothorax and comparison with findings in nor-mal age-matched controls. Pediatr Radiol 2007;37:879-84. [Crossref] [PubMed]
- Soccorso G, Anbarasan R, Singh M, et al. Management of large primary sponta-neous pneumothorax in children: radiological guidance, surgical intervention and proposed guideline. Pediatr Surg Int 2015;31:1139-44. [Crossref] [PubMed]
- Choudhary AK, Sellars ME, Wallis C, et al. Primary spontaneous pneumothorax in children: the role of CT in guiding management. Clin Radiol 2005;60:508-11. [Crossref] [PubMed]
- Lee KH, Kim KW, Kim EY, et al. Detection of blebs and bullae in patients with primary spontaneous pneumothorax by multi-detector CT reconstruction using different slice thicknesses. J Med Imaging Radiat Oncol 2014;58:663-7. [Crossref] [PubMed]
- Chiu CY, Chen TP, Wang CJ, et al. Factors associated with proceeding to surgical intervention and recurrence of primary spontaneous pneumothorax in adolescent patients. Eur J Pediatr 2014;173:1483-90. [Crossref] [PubMed]
- Laituri CA, Valusek PA, Rivard DC, et al. The utility of computed tomography in the management of patients with spontaneous pneumothorax. J Pediatr Surg 2011;46:1523-5. [Crossref] [PubMed]
- Voisin F, Sohier L, Rochas Y, et al. Ambulatory management of large spontaneous pneumothorax with pigtail catheters. Ann Emerg Med 2014;64:222-8. [Crossref] [PubMed]
- Sayar A, Kök A, Citak N, et al. A. Size of pneumothorax can be a new indication for surgical treatment in primary spontaneous pneumothorax: a prospective study. Ann Thorac Cardiovasc Surg 2014;20:192-7. [Crossref] [PubMed]
- Qureshi FG, Sandulache VC, Richardson W, et al. Primary vs delayed surgery for spontaneous pneumothorax in children: which is better? J Pediatr Surg 2005;40:166-9. [Crossref] [PubMed]
- Chambers A, Scarci M. In patients with first-episode primary spontaneous pneumothorax is video-assisted thoracoscopic surgery superior to tube thoracostomy alone in terms of time to resolution of pneumothorax and incidence of recurrence? Interact Cardiovasc Thorac Surg 2009;9:1003-8. [Crossref] [PubMed]
- Herrmann D, Klapdor B, Ewig S, et al. Initial management of primary spontaneous pneumothorax with video-assisted thoracoscopic surgery: a 10-year experience. Eur J Cardiothorac Surg 2016;49:854-9. [Crossref] [PubMed]
- Morimoto T, Fukui T, Koyama H, et al. Optimal strategy for the first episode of primary spontaneous pneumothorax in young men. A decision analysis. J Gen Intern Med 2002;17:193-202. [Crossref] [PubMed]
- Williams K, Lautz TB, Leon AH, et al. Optimal timing of video-assisted thoracoscopic surgery for primary spontaneous pneumothorax in children. J Pediatr Surg 2018;53:1858-61. [Crossref] [PubMed]
- Margolis M, Gharagozloo F, Tempesta B, et al. Video-assisted thoracic surgical treatment of initial spontaneous pneumothorax in young patients. Ann Thorac Surg 2003;76:1661-3; discussion 1663-4.
- Ganesalingam R, O'Neil RA, Shadbolt B, et al. Radiological predictors of recurrent primary spontaneous pneumothorax following nonsurgical management. Heart Lung Circ 2010;19:606-10. [Crossref] [PubMed]
- Kim YP, Haam SJ, Lee S, et al. Effectiveness of Ambulatory Tru-Close Thoracic Vent for the Outpatient Management of Pneumothorax: A Prospective Pilot Study. Korean J Radiol 2017;18:519-25. [Crossref] [PubMed]
- Hassani B, Foote J, Borgundvaag B. Outpatient management of primary spontaneous pneumothorax in the emergency department of a community hospital using a small-bore catheter and a Heimlich valve. Acad Emerg Med 2009;16:513-8. [Crossref] [PubMed]
- O'Lone E, Elphick HE, Robinson PJ. Spontaneous pneumothorax in children: when is invasive treatment indicated? Pediatr Pulmonol 2008;43:41-6. [Crossref] [PubMed]
- Park CB, Moon MH, Jeon HW, et al. Does oxygen therapy increase the resolution rate of primary spontaneous pneumothorax? J Thorac Dis 2017;9:5239-43. [Crossref] [PubMed]
- Camuset J, Laganier J, Brugière O, et al. Needle aspiration as first-line management of primary spontaneous pneumothorax. Presse Med 2006;35:765-8. [Crossref] [PubMed]
- Noppen M, Alexander P, Driesen P, et al. Manual aspiration versus chest tube drainage in first episodes of primary spontaneous pneumothorax: a multicenter, prospective, randomized pilot study. Am J Respir Crit Care Med 2002;165:1240-4. [Crossref] [PubMed]
- Kuo HC, Lin YJ, Huang CF, et al. Small-bore pigtail catheters for the treatment of primary spontaneous pneumothorax in young adolescents. Emerg Med J 2013;30:e17 [Crossref] [PubMed]
- Kelly AM, Kerr D, Clooney M. Outcomes of emergency department patients treated for primary spontaneous pneumothorax. Chest 2008;134:1033-6. [Crossref] [PubMed]
- Dull KE, Fleisher GR. Pigtail catheters versus large-bore chest tubes for pneumothoraces in children treated in the emergency department. Pediatr Emerg Care 2002;18:265-7. [Crossref] [PubMed]
- Riber SS, Riber LP, Olesen WH, et al. The influence of chest tube size and position in primary spontaneous pneumothorax. J Thorac Dis 2017;9:327-32. [Crossref] [PubMed]
- Tschopp JM, Bintcliffe O, Astoul P, et al. ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. Eur Respir J 2015;46:321-35. [Crossref] [PubMed]
- Levi JF, Kleinmann P, Riquet M, et al. Percutaneous parietal pleurectomy for recurrent spontaneous pneumothorax. Lancet 1990;336:1577-8. [Crossref] [PubMed]
- Freixinet J, Canalis E, Rivas JJ, et al. Surgical treatment of primary spontaneous pneumothorax with video-assisted thoracic surgery. Eur Respir J 1997;10:409-11. [Crossref] [PubMed]
- Naunheim KS, Mack MJ, Hazelrigg SR, et al. Safety and efficacy of video-assisted thoracic surgical techniques for the treatment of spontaneous pneumothorax. J Thorac Cardiovasc Surg 1995;109:1198-203; discussion 1203-4. [Crossref] [PubMed]
- Ayed AK, Al-Din HJ. The results of thoracoscopic surgery for primary spontaneous pneumothorax. Chest 2000;118:235-8. [Crossref] [PubMed]
- Körner H, Andersen KS, Stangeland L, et al. Surgical treatment of spontaneous pneumothorax by wedge resection without pleurodesis or pleurectomy. Eur J Cardiothorac Surg 1996;10:656-9. [Crossref] [PubMed]
- Massard G, Thomas P, Wihlm JM. Minimally invasive management for first and recurrent pneumothorax. Ann Thorac Surg 1998;66:592-9. [Crossref] [PubMed]
- Ayed AK, Jamal Al-Din H. Video-Assisted Thoracoscopy versus Thoracotomy for Primary Spontaneous Pneumothorax: A Randomized Controlled Trial. Med Principles Pract 2000;9:113-8. [Crossref]
- Waller DA, Forty J, Morritt GN. Video-assisted thoracoscopic surgery versus thoracotomy for spontaneous pneumothorax. Ann Thorac Surg 1994;58:372-6; discussion 376-7. [Crossref] [PubMed]
- Bialas RC, Weiner TM, Phillips JD. Video-assisted thoracic surgery for primary spontaneous pneumothorax in children: is there an optimal technique? J Pediatr Surg 2008;43:2151-5. [Crossref] [PubMed]
- Cho KD, Park CB, Cho MS, et al. Modification of thoracoscopic surgery for spontaneous pneumothorax. Asian Cardiovasc Thorac Ann 2006;14:472-5. [Crossref] [PubMed]
- Yamazaki K, Haratake N, Shikada Y, et al. Initial Experience of Single-Incision Thoracoscopic Surgery for 100 Patients with Primary Spontaneous Pneumothorax. Ann Thorac Cardiovasc Surg 2015;21:513-6. [Crossref] [PubMed]
- Yang HC, Kim S, Yum S, et al. Learning curve of single-incision thoracoscopic surgery for primary spontaneous pneumothorax. Surg Endosc 2017;31:1680-7. [Crossref] [PubMed]
- Ocakcioglu I, Alpay L, Demir M, et al. Is single port enough in minimally surgery for pneumothorax? Surg Endosc 2016;30:59-64. [Crossref] [PubMed]
- Prasad R, Arthur LG, Timmapuri SJ, et al. Early experience with single-incision thoracoscopic surgery in the pediatric population. J Laparoendosc Adv Surg Tech A 2011;21:189-92. [Crossref] [PubMed]
- Song IH, Yum S, Choi W, et al. Clinical application of single incision thoracoscopic surgery: early experience of 264 cases. J Cardiothorac Surg 2014;9:44. [Crossref] [PubMed]
- Chung PH, Wong KK, Lan LC, et al. Thoracoscopic bullectomy for primary spontaneous pneumothorax in pediatric patients. Pediatr Surg Int 2009;25:763-6. [Crossref] [PubMed]
- Casadio C, Rena O, Giobbe R, et al. Stapler blebectomy and pleural abrasion by video-assisted thoracoscopy for spontaneous pneumothorax. J Cardiovasc Surg (Torino) 2002;43:259-62. [PubMed]
- Stringel G, Amin NS, Dozor AJ. Video-assisted thoracoscopy in the management of recurrent spontaneous pneumothorax in the pediatric population. JSLS 1999;3:113-6. [PubMed]
- Obuchi T, Yoshida Y, Wakahara JI, et al. Pneumothorax in teenagers: reducing recurrence through resection of superior segment of lower lobe. J Thorac Dis 2018;10:3507-11. [Crossref] [PubMed]
- Takahashi R. Evaluation of Spontaneous Pneumothorax Surgeries: A 16-Year Experience in Japan. Surg Res Pract 2016;2016:7025793 [Crossref] [PubMed]
- Torre M, Grassi M, Nerli FP, et al. Nd-YAG laser pleurodesis via thoracoscopy. Endoscopic therapy in spontaneous pneumothorax Nd-YAG laser pleurodesis. Chest 1994;106:338-41. [Crossref] [PubMed]
- Li Z, Chen L, Wang J, et al. A single institution experience using the LigaSure vessel sealing system in video-assisted thoracoscopic surgery for primary spon-taneous pneumothorax. J Biomed Res 2014;28:494-7. [PubMed]
- Linchevskyy O, Makarov A, Getman V. Lung sealing using the tissue-welding technology in spontaneous pneumothorax. Eur J Cardiothorac Surg 2010;37:1126-8. [Crossref] [PubMed]
- Lee S, Kim HR, Cho SKorean Pneumothorax Study Group, et al. Staple line coverage after bullectomy for primary spontaneous pneumothorax: a randomized trial. Ann Thorac Surg 2014;98:2005-11. [Crossref] [PubMed]
- Haraguchi S, Koizumi K, Mikami I, et al. Staple line coverage with a polyglycolic acid sheet plus pleural abrasion by thoracoscopic surgery for primary spontaneous pneumothorax in young patients. J Nippon Med Sch 2012;79:139-42. [Crossref] [PubMed]
- Cho S, Jheon S, Kim D, et al. Results of repeated video-assisted thoracic surgery for recurrent pneumothorax after primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2018;53:857-61. [Crossref] [PubMed]
- Vuong NL, Elshafay A, Thao LP, et al. Efficacy of treatments in primary spontaneous pneumothorax: A systematic review and network meta-analysis of randomized clinical trials. Respir Med 2018;137:152-66. [Crossref] [PubMed]
- Chan P, Clarke P, Daniel FJ, et al. Efficacy study of video-assisted thoracoscopic surgery pleurodesis for spontaneous pneumothorax. Ann Thorac Surg 2001;71:452-4. [Crossref] [PubMed]
- Rena O, Massera F, Papalia E, et al. Surgical pleurodesis for Vanderschueren's stage III primary spontaneous pneumothorax. Eur Respir J 2008;31:837-41. [Crossref] [PubMed]
- Gossot D, Galetta D, Stern JB, et al. Results of thoracoscopic pleural abrasion for primary spontaneous pneumothorax. Surg Endosc 2004;18:466-71. [Crossref] [PubMed]
- Maier A, Anegg U, Renner H, et al. Four-year experience with pleural abrasion using a rotating brush during video-assisted thoracoscopy. Surg Endosc 2000;14:75-8. [Crossref] [PubMed]
- Bobbio A, Ampollini L, Internullo E, et al. Thoracoscopic parietal pleural argon beam coagulation versus pleural abrasion in the treatment of primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2006;29:6-8. [Crossref] [PubMed]
- Dubois L, Malthaner RA. Video-assisted thoracoscopic bullectomy and talc poudrage for spontaneous pneumothoraces: effect on short-term lung function. J Thorac Cardiovasc Surg 2010;140:1272-5. [Crossref] [PubMed]
- Chen JS, Chan WK, Tsai KT, et al. Simple aspiration and drainage and intrapleural minocycline pleurodesis versus simple aspiration and drainage for the initial treatment of primary spontaneous pneumothorax: an open-label, parallel-group, prospective, randomised, controlled trial. Lancet 2013;381:1277-82. [Crossref] [PubMed]
- Ling ZG, Wu YB, Ming MY, et al. The effect of pleural abrasion on the treatment of primary spontaneous pneumothorax: a systematic review of randomized controlled trials. PLoS One 2015;10:e0127857 [Crossref] [PubMed]
- Lillegard JB, Kennedy RD, Ishitani MB, et al. Autologous blood patch for persistent air leak in children. J Pediatr Surg 2013;48:1862-6. [Crossref] [PubMed]
- Cobanoglu U, Melek M, Edirne Y. Autologous blood pleurodesis: A good choice in patients with persistent air leak. Ann Thorac Med 2009;4:182-6. [Crossref] [PubMed]
- Pathak V, Quinn C, Zhou C, et al. Use of autologous blood patch for prolonged air leak in spontaneous pneumothoraces in the adolescent population. Lung India 2018;35:328-31. [Crossref] [PubMed]
- Alayouty HD, Hasan TM, Alhadad ZA, et al. Mechanical versus chemical pleurodesis for management of primary spontaneous pneumothorax evaluated with thoracic echography. Interact Cardiovasc Thorac Surg 2011;13:475-9. [Crossref] [PubMed]
- Choi SY, Du Kim Y, Kim DY, et al. Influence of lung resection volume on risk of primary spontaneous pneumothorax recurrence. J Thorac Dis 2018;10:1622-7. [Crossref] [PubMed]
- Young Choi S, Beom Park C, Wha Song S, et al. What factors predict recurrence after an initial episode of primary spontaneous pneumothorax in children? Ann Thorac Cardiovasc Surg 2014;20:961-7. [Crossref] [PubMed]
- Uramoto H, Shimokawa H, Tanaka F. What factors predict recurrence of a spontaneous pneumothorax? J Cardiothorac Surg 2012;7:112. [Crossref] [PubMed]
- Williams K, Oyetunji TA, Hsuing G, et al. Spontaneous Pneumothorax in Children: National Management Strategies and Outcomes. J Laparoendosc Adv Surg Tech A 2018;28:218-22. [Crossref] [PubMed]
- Cardillo G, Carleo F, Carbone L, et al. Long-term lung function following video-thoracoscopic talc poudrage for primary spontaneous recurrent pneumothorax. Eur J Cardiothorac Surg 2007;31:802-5. [Crossref] [PubMed]
- Grewal H, Jackson RJ, Dunton RF, et al. Video-assisted thoracic surgery is safe and effective in the treatment of spontaneous pneumothorax in children. Pediatric Endosurgery and Innovative Techniques 2001;5:365-70. [Crossref]
- Shaikhrezai K, Thompson AI, Parkin C, et al. Video-assisted thoracoscopic surgery management of spontaneous pneumothorax--long-term results. Eur J Cardiothorac Surg 2011;40:120-3. [Crossref] [PubMed]
- Doddoli C, Barlési F, Fraticelli A, et al. Video-assisted thoracoscopic management of recurrent primary spontaneous pneumothorax after prior talc pleurodesis: a feasible, safe and efficient treatment option. Eur J Cardiothorac Surg 2004;26:889-92. [Crossref] [PubMed]
- Imperatori A, Rotolo N, Spagnoletti M, et al. Risk factors for postoperative recurrence of spontaneous pneumothorax treated by video-assisted thoracoscopic surgery. Interact Cardiovasc Thorac Surg 2015;20:647-51; discussion 651-2. [Crossref] [PubMed]
- Bintcliffe OJ, Hallifax RJ, Edey A, et al. Spontaneous pneumothorax: time to rethink management? Lancet Respir Med 2015;3:578-88. [Crossref] [PubMed]
- Karpman C, Aughenbaugh GL, Ryu JH. Pneumothorax and bullae in Marfan syndrome. Respiration 2011;82:219-24. [Crossref] [PubMed]
- Weissberg D, Refaely Y. Pneumothorax: experience with 1,199 patients. Chest 2000;117:1279-85. [Crossref] [PubMed]
- Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med 2007;175:1044-53. [Crossref] [PubMed]
- Loung RP, Cooney M, Fallon EM, et al. Pneumothorax in a young man with anorexia nervosa. Int J Eat Disord 2016;49:895-8. [Crossref] [PubMed]
- Tan J, Yang Y, Zhong J, et al. Association Between BMI and Recurrence of Primary Spontaneous Pneumothorax. World J Surg 2017;41:1274-80. [Crossref] [PubMed]
- Baumann MH, Strange C. Treatment of spontaneous pneumothorax: a more aggressive approach? Chest 1997;112:789-804. [Crossref] [PubMed]
- Brims FJ, Maskell N. Ambulatory treatment in the management of pneumothorax: a systematic review of the literature. Thorax 2013;68:664-9. [Crossref] [PubMed]
- Kepka S, Dalphin JC, Pretalli JB, et al. How spontaneous pneumothorax is managed in emergency departments: a French multicentre descriptive study. BMC Emerg Med 2019;19:4. [Crossref] [PubMed]
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Furia S, Breda C. Primary spontaneous pneumothorax in children and adolescents: a systematic review . Pediatr Med 2019;2:12.



