Lung transplantation in adolescents
Introduction
Adolescence classically defines the years between ages 13 and 19 and can be considered the transitional period from infancy to maturity. This transitional age is a time of both confusion and discovery, bringing up concerns regarding identity, independence, sexuality and social life. In the modern era, social changes have resulted in the lengthening of the timeline for transition to maturity, prolonging the end of adolescence. The indication for lung transplantation in adolescents is progressive respiratory failure, just like in adults. However, this procedure could be particularly challenging for young patients because it requires complex medications, physical therapy schedules, dietary regimens, demanding medical and invasive follow-up. Despite the recipients’ young age and limited comorbidities, analyses of heart, liver and renal transplantation cohorts documented a correlation between adolescent age at transplantation and poorer clinical outcomes compared to older recipients (1,2). The increased risk for non-adherence, associated with a vigorous immune system, could justify the higher frequencies of acute and chronic rejection in adolescents (3).
This study describes a 10 years’ experience of lung transplantation at a single center in Italy comparing adolescent with older recipients.
Methods
The study was approved by the local ethics committee (749_2016bis) and all participants gave written informed consent.
The same surgical team transplanted all the patients without substantial technical differences over time. After transplantation, all patients received triple-drug immunosuppressive therapy with tacrolimus, azathioprine and prednisolone. None of the patients received induction therapy. After discharge, patients returned for follow-up visits according to a scheduled protocol or when clinically indicated. Each clinical check included pulmonary functional tests and blood examinations; immunosuppressive agents were also monitored to assess therapeutic levels. Patients underwent routine surveillance bronchoscopy with transbronchial lung biopsies at 3, 6 and 12 months after transplantation; additional procedures were scheduled in case of signs and/or symptoms of possible lung allograft dysfunction.
We extracted data from our institutional database dedicated to lung transplantation. This was a prospective observational study on consecutive patients who underwent lung transplantation from January 1st 2008 to March 1st 2019. Considering that adolescence is prolonging in western countries and following the choice done in a recent analysis of the International Society for Heart and Lung Transplantation Registry (4), we divided the patients’ cohort into 2 groups, based on the age of the recipient at transplantation: Group A (adolescent, 10–24 years) and Group C (control cohort, ≥25 years). These two groups were then compared in terms of chronic lung allograft dysfunction (CLAD) and survival.
We identified the onset of CLAD when a patient presented a persistent decline in forced expiratory volume in 1 second greater than 20% compared to the mean of the two best postoperative values, in the absence of other known causes for lung allograft dysfunction, including acute infection and/or acute rejection (5). We did not classify the subtypes of CLAD for the purpose of this study. Overall survival and CLAD-free survival data were carefully checked and collected. Data were censored at death or retransplantation; patients undergoing retransplantation were counted as two distinct cases.
We described the baseline clinical characteristics of the 2 groups by standard descriptive statistics; data were presented as mean and standard deviation or median and 95% confidence interval (CI) for continuous variables; for categorical variables, absolute number and percentage were used. Categorical variables were compared among groups using the chi-square test or Fisher’s exact test, whereas continuous variables were compared using the Mann-Whitney test for independent samples. Overall survival and CLAD-free survival analyses were performed and stratified by age group using the Kaplan-Meier test; survival rate curves were compared using the log-rank test. To determine risk factors for mortality and CLAD occurrence, we used univariate, followed by multivariate backward Cox proportional-hazard regression models for each of the end-points. All analyses were performed using MedCalc 18.2 (MedCalc Software, Ostend, Belgium) software.
Results
We transplanted 223 patients from January 1st, 2008 to March 1st, 2019; adolescents (Group A) made up 14.8% of the cohort. Table 1 summarizes essential clinical data. Underlying diseases were unbalanced between groups; cystic fibrosis (CF) was the most common indication for transplantation in Group A (94% vs. 41%). A tendency for lower prevalence of male gender in Group A was evident. Adolescents were more frequently bridged to transplantation with extracorporeal membrane oxygenation (ECMO) support (21% vs. 13%) but the difference was not statistically significant. The entire Group A received a bilateral transplantation, whereas 24% of Group C received a single lung (P<0.001). The median Oto score, the number of donors after circulatory death and the number of marginal grafts treated with ex vivo lung perfusion was well balanced between the two groups.
Table 1
| Variables | Adolescents (n=33) | Controls (n=190) | P |
|---|---|---|---|
| Age, mean (SD) | 20.0 (2.8) | 46.8 (13.5) | |
| Male, % | 36.4 | 53.7 | 0.066 |
| LAS, median (95% CI) | 39.9 (35.1–44.9) | 39.8 (38.0–41.3) | 0.716 |
| Underling diseases, n (%) | <0.001 | ||
| Cystic fibrosis | 31 (93.9) | 78 (41.1) | |
| COPD | 0 (0.0) | 19 (10.0) | |
| IPF | 0 (0.0) | 66 (34.7) | |
| Retransplantation | 2 (6.1) | 4 (2.1) | |
| Other | 0 (0.0) | 23 (12.1) | |
| ECMO bridge, n (%) | 7 (21.2) | 25 (13.2) | 0.224 |
| Bilateral lung transplantation, n (%) | 33 (100.0) | 144 (75.8) | <0.001 |
| Lobar transplantation, n (%) | 1 (3.0) | 9 (4.7) | 1.000 |
| EVLP, n (%) | 5 (15.2) | 31 (16.3) | 1.000 |
| DCD donor, n (%) | 1 (3.0) | 8 (4.2) | 1.000 |
| Oto score, median (95% CI) | 3 (1 to 5) | 3 (3 to 4) | 0.857 |
LAS, lung allocation score; COPD, chronic obstructive pulmonary disease; IPF, idiopathic pulmonary fibrosis; ECMO, extracorporeal membrane oxygenation; DCD, donation after circulatory death.
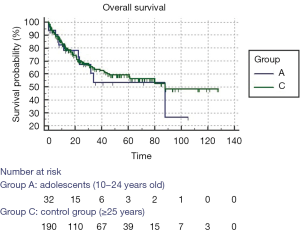
Figure 1 shows the overall survival curves stratified by age group; the curves do not show any statistically significant difference (log-rank test, P=0.568). The results of univariate Cox proportional-hazards regression for overall survival is shown in Table 2. Multivariate analysis examined the effect of previous significant covariates (cystic fibrosis, age, single lung transplantation and Oto score) on mortality by backward Cox proportional-hazards regression; diagnosis of cystic fibrosis had a hazard ratio of 0.45 (95% CI: 0.2643–0.7830; P=0.0045) and Oto score had a hazard ratio of 1.13 (95% CI: 1.0199–1.2462; P=0.019).
Table 2
| Covariate | P | Exp(b) | 95% CI |
|---|---|---|---|
| Not cystic fibrosis disease | 0.0002 | 2.5182 | 1.5464 to 4.1007 |
| Age | 0.0066 | 1.0214 | 1.0059 to 1.0372 |
| Male gender | 0.8202 | 1.0529 | 0.6752 to 1.6417 |
| Group A | 0.5693 | 1.2040 | 0.6353 to 2.2820 |
| LAS | 0.6152 | 0.9961 | 0.9811 to 1.0114 |
| Single lung transplantation | 0.0009 | 2.1696 | 1.3739 to 3.4262 |
| Lobar transplantation | 0.5414 | 0.6449 | 0.1578 to 2.6354 |
| Oto score | 0.0068 | 1.1454 | 1.0381 to 1.2639 |
| EVLP | 0.4344 | 1.2791 | 0.6900 to 2.3713 |
| Diagnosis of CLAD | 0.3269 | 0.7622 | 0.4429 to 1.3117 |
LAS, lung allocation score; EVLP,
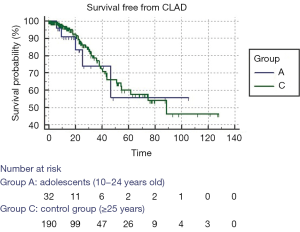
Figure 2 shows the Kaplan-Meier test for CLAD-free survival; the two age groups had similar curves and log-rank test was not significant (P=0.695).
Discussion
A British study published in 2013 identified patients aged 16–20 years as a patients’ cohort at high risk for mortality after heart or lung transplantation (6). Subsequently, Foster and collaborators evaluated 17,181 patients recorded in the Scientific Registry of Transplant Recipients who received liver transplantation from 1988 to 2013; the authors found that patients had a high risk of graft failure during adolescence and young adulthood; this risk was greater than in patients belonging to other age cohorts (2). The maturation of the immune system during adolescence could justify a change in immune response to transplantation. The ability to tolerate an allograft is probably influenced by the T cells present at the time of surgery; with age, these cells are increasingly activated, leading to lower thresholds for alloantigen‐stimulated activation (7). In addition, adherence for adolescents and young adults is affected by emotional changes typical of this age period.
Recently, Paraskeva et al. published an analysis of the International Society for Heart and Lung Transplantation Registry focused on lung transplantation in adolescents (4). Such analysis included 2,319 adolescents divided in 3 age cohorts. Adolescents aged 10–24 years had worse survival than younger children and older adults; in particular, the 15- to 19-year-old cohort had the poorest outcomes.
The survival analysis of our lung-transplanted adolescents did not demonstrate a significant difference with the cohort of adult patients. The reason for this homogeneity can be found in at least three hypotheses. One possibility is that it could be simply linked to a problem of limited sample size. Another hypothesis is a geographical matter; the International Society for Heart and Lung Transplantation Registry collects mainly patients from USA whereas papers with good results from adolescent cohorts were frequently from other countries (8-10). A third more speculative hypothesis may lie in the fact that almost all of our adolescent recipients consisted of patients suffering from cystic fibrosis. It is well known that monocyte-macrophages in cystic fibrosis are metabolically hyperactive and hypersecretory, thus accelerating the development of a chronic inflammation by elevated concentrations of pro-inflammatory cytokines such as interleukin-1, 6, 8 and tumor necrosis factor-alpha (11). It is possible that colonization by multi-resistant bacteria, along with inflammatory milieu, give the immune system the possibility of developing completely at an early age before transplantation, thus eliminating a change in the asset of the alloantigen response during adolescence.
Multivariate analysis for overall survival identified donors’ quality (defined by Oto score) as a risk factor for increased mortality in our patients (hazard ratio 1.13). The quality score proposed by Oto and collaborators in 2007 was elaborated to predict early outcome after lung transplantation; such a simple score demonstrated a good correlation with overall survival in our patients (12). Multivariate analysis also highlighted that diagnosis of cystic fibrosis seems to guarantee a better outcome independently from age class (hazard ratio 0.69). Indeed, patients with cystic fibrosis have a superior survival after lung transplantation compared to other indications, as recently documented by the registry of the International Society for Heart and Lung Transplantation (13). To reduce both the time on waiting list for lung transplantation and waiting list mortality, especially for small size candidates, we implemented the down-sizing of larger adult lungs (14); this procedure did not affect survival in the univariate and multivariate analysis. In addition, we implemented the use of grafts from donation after circulatory death to provide supplementary lung transplantation opportunities (15); no detrimental effect on survival was observed in this case too.
It is suspected that CLAD is prompted by Pseudomonas and Aspergillus infections that commonly colonize the allograft after transplantation in patients with cystic fibrosis (16). Even though our adolescent cohort included 94% of patients with cystic fibrosis, the analysis of CLAD-free survival failed to demonstrate any difference between our adolescent and adult cohort. It should be underlined that longitudinal changes in bacterial flora have been described after lung transplantation; whether change in microbiota over time is more important than individual pathogens in the development of CLAD is discussed. It has already been demonstrated that the risk of CLAD is significantly higher in patients with new Pseudomonas colonization versus those with pre-transplant germs; thus, it is possible that acquired Pseudomonas is more immunogenic than graft re-colonization by previous microbiota (17). In addition, an alteration of a previously established equilibrium and ecological mixture with the onset of low microbial diversity could trigger inflammatory response and pneumonia (18).
In conclusion, our data show that lung transplantation can be successfully undertaken in adolescent patients with end-stage lung disease; overall survival and CLAD-free survival resulted similar to those observed in older patients in our study population. Attention should be paid to donor quality because poor graft could affect long term outcome.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Paolo Scanagatta) for the series “Pediatric Thoracic Surgery” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.05.04). The series "Pediatric Thoracic Surgery" was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethics committee (749_2016bis) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dharnidharka VR, Lamb KE, Zheng J, et al. Across all solid organs, adolescent age recipients have worse transplant organ survival than younger age children: a US national registry analysis. Pediatr Transplant 2015;19:471-6. [Crossref] [PubMed]
- Foster BJ, Dahhou M, Zhang X, et al. High risk of liver allograft failure during late adolescence and young adulthood. Transplantation 2016;100:577-84. [Crossref] [PubMed]
- Hsu DT. Biological and psychological differences in the child and adolescent transplant recipient. Pediatr Transplant 2005;9:416-21. [Crossref] [PubMed]
- Paraskeva MA, Edwards LB, Levvey B, et al. Outcomes of adolescent recipients after lung transplantation: An analysis of the International Society for Heart and Lung Transplantation Registry. J Heart Lung Transplant 2018;37:323-31. [Crossref] [PubMed]
- Van Herck A, Verleden SE, Sacreas A, et al. Validation of a post-transplant chronic lung allograft dysfunction classification system. J Heart Lung Transplant 2019;38:166-73. [Crossref] [PubMed]
- Wray J, Sugarman H, Davis L, et al. Adolescence and transition to adult services: are these risky times for heart and/or lung recipients? J Heart Lung Transplant 2013;32:S194-5. [Crossref]
- Dhanireddy KK, Maniscalco J, Kirk AD. Is tolerance induction the answer to adolescent non-adherence? Pediatr Transplant 2005;9:357-63. [Crossref] [PubMed]
- Alvarez A, Algar FJ, Santos F, et al. Pediatric lung transplantation. Transplant Proc 2005;37:1519-22. [Crossref] [PubMed]
- Benden C, Harpur-Sinclair O, Ranasinghe AS, et al. Surveillance bronchoscopy in children during the first year after lung transplantation: Is it worth it? Thorax 2007;62:57-61. [Crossref] [PubMed]
- Morton JM, Malouf MA, Plit ML, et al. Successful lung transplantation for adolescents at a hospital for adults. Med J Aust 2007;187:278-82. [PubMed]
- Courtney JM, Ennis M, Elborn JS. Cytokines and inflammatory mediators in cystic fibrosis. J Cyst Fibros 2004;3:223-31. [Crossref] [PubMed]
- Oto T, Levvey BJ, Whitford H, et al. Feasibility and utility of a lung donor score: correlation with early post-transplant outcomes. Ann Thorac Surg 2007;83:257-63. [Crossref] [PubMed]
- Chambers DC, Yusen RD, Cherikh WS, et al. The registry of the international society for heart and lung transplantation: thirty-fourth adult lung and heart-lung transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant 2017;36:1047-59. [Crossref] [PubMed]
- Mendogni P, Palleschi A, Tosi D, et al. Lobar Lung Transplantation From Deceased Donor: Monocentric Experience. Transplant Proc 2017;49:682-5. [Crossref] [PubMed]
- Valenza F, Citerio G, Palleschi A, et al. Successful Transplantation of Lungs From an Uncontrolled Donor After Circulatory Death Preserved In Situ by Alveolar Recruitment Maneuvers and Assessed by Ex Vivo Lung Perfusion. Am J Transplant 2016;16:1312-8. [Crossref] [PubMed]
- Westall GP, Paraskeva MA, Snell GI. Antibody-mediated rejection. Curr Opin Organ Transplant 2015;20:492-7. [Crossref] [PubMed]
- Botha P, Archer L, Anderson RL, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation 2008;85:771-4. [Crossref] [PubMed]
- Willner D, Haynes MR, Furlan M, et al. Spatial distribution of microbial communities in the cystic fibrosis lung. ISME J 2012;6:471-4. [Crossref] [PubMed]
Cite this article as: Nosotti M, Morlacchi LC, Rossetti V, Musso V, Rosso L. Lung transplantation in adolescents. Pediatr Med 2019;2:22.