
Dysmenorrhea in adolescents
Introduction
Dysmenorrhea, or pain with menses, is a common complaint in adolescents. The estimated prevalence of dysmenorrhea ranges from 45% to 93% of women of reproductive age, with adolescents having the highest rates of dysmenorrhea (1-3). An estimated 15% of adolescent females describe their pain as severe, impacting on their quality of life. These adolescents can miss 1 to 3 days of school per menstrual cycle. Studies have noted that dysmenorrhea can lead to lower academic performance, and poor quality of sleep, resulting in mood changes, such as anxiety and depression (4-8).
Primary dysmenorrhea
The majority of cases of dysmenorrhea in adolescents are primary. Primary dysmenorrhea is defined as lower abdominal menstrual cramping in the absence of any other pelvic disease or abnormality. Primary dysmenorrhea is associated with normal ovulatory cycles. Onset of dysmenorrhea is typically 6 to 12 months after menarche, with peak prevalence occurring in the late teens or early twenties.
Etiology
The etiology of primary dysmenorrhea has been well documented. There is an increase in fatty-acid build up in the phospholipids of the cell membranes after ovulation. After progesterone withdrawal, the phospholipids from the dead cell membranes are converted to arachidonic acid (AA), which is metabolized by lipoxygenase and cyclooxygenase (COX), beginning the production of leukotrienes (LTs) and prostaglandins (PGs) respectively. PGF2 alpha and PGE2, are the PGs responsible for mediating the pain and other symptoms associated with dysmenorrhea. PGF2 alpha mediates pain sensation and stimulates smooth muscle contraction. While PGE2 potentiates platelet disaggregation and vasodilation, producing cramping and systemic symptoms such as nausea, vomiting, bloating, and headaches. The PGF2 alpha and COX metabolite of AA, cause potent vasoconstriction and myometrial contractions leading to uterine ischemia and pain (9,10).
Several studies have examined the role of PGF2 alpha in dysmenorrhea and found that PGF2 alpha levels were 2 to 4 times higher in dysmenorrheic women compared to eumenorrheic women. The intensity of the menstrual cramps is directly related to the amount of PGF2 alpha released (9,10). There have been fewer investigations into the role of LTs in uterine cramping. A few studies of LTs, indicate that high values of LTs were present in women who experience dysmenorrhea (11,12). Other mechanisms may be involved in uterine contractility and relaxation, however further studies are needed.
Clinical presentation
Lower abdominal pain or pelvic pain is a typical presenting symptom of dysmenorrhea in adolescents. Headache, nausea and vomiting can accompany the pain as well. Pain can radiate to the thighs or back. The pain typically presents at the onset of menstrual flow and can last anywhere from 8 to 72 hours. Pain can start 1 to 2 days prior to start of menses and may last up to 4 days into menses. Pain occurring outside of menses may be due to secondary causes and would warrant further investigation. Severity of pain and associated symptoms positively correlates with the onset of ovulatory cycles and with increased duration and amount of menstrual flow (13). Other risk factors associated with primary dysmenorrhea include low fish consumption and cigarette smoking (13,14).
Risk factors
Dysmenorrhea has been associated with several risk factors. Studies have noted that younger age of menses has been associated with dysmenorrhea. One study found that women younger than 25 years were more than twice at risk of reporting moderate to severe pain compared with those aged 25–34 years (2,15-17). Family history of dysmenorrhea was noted to have a strong association with reporting of menstrual pain (18,19). The association between cigarette smoking and dysmenorrhea was mixed. One longitudinal and two cross-sectional studies did not detect a significant relationship, while one cross-sectional study did observe a relationship for dysmenorrhea amongst smokers (2,18,20).
The presence of heavy menses or irregular menses was associated with an increased risk for dysmenorrhea (15,18). Parity or number of live births had a negative relationship with dysmenorrhea (2). Fruit and vegetable intake, and sociodemographic factors such as years of education, marital status, and area of residence, were not associated with dysmenorrhea (21-23). Stress and depression were associated with dysmenorrhea in several studies. Reports of an increase in stress or being depressed increased the reporting of pain with menses (20,24). It is important to note that there is a lack of longitudinal data on the natural history of dysmenorrhea and the possible modifiable factors associated with it. Further research is needed that may contribute to preventative solutions.
Evaluation
When presented with an adolescent complaining of pelvic pain, the provider should obtain a thorough history of the symptoms. History should include a menstrual history; age of menarche, location of pain, duration of pain, degree of impairment in their daily activities (i.e., missed days of school or activities), and any treatments that they may have tried in the past. All pain may not be primary dysmenorrhea and it is important to take a social history as well. History of pain with sex, or a past medical history of a sexually transmitted infection (STI) should be obtained. Providers should ask about whether the patient is engaging in cigarette use or other nicotine use, any recreational drug use and their mood. History of physical or sexual abuse should be obtained as well.
Physical examination should include an examination of the external genitalia. A pelvic exam may be deferred if the history is most consistent with primary dysmenorrhea or the adolescent is not sexually active. Anatomic abnormalities that can be seen on visual inspection include hymen abnormalities, such as an imperforate hymen or a fenestrated hymen. The vaginal septum can be identified with the insertion of a cotton tipped swab into the vaginal canal.
Sexually active adolescents should be screened for STIs and pregnancy. A urinalysis can be done to rule out a urinary tract infection. A complete blood count (CBC), C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) may be obtained when considering pelvic inflammatory disease (PID) or other inflammatory process. Imaging, such as ultrasound or magnetic resonance imaging (MRI), can help identify any uterine or pelvic organ abnormalities. Laparoscopy should be considered when there is no etiology identified and there is failure of treatment with first-line medications.
Treatment options
Non-steroidal anti-inflammatory drugs (NSAIDs) are considered the first-line medication for the treatment of dysmenorrhea. A Cochrane review of 73 randomized control trials demonstrated strong evidence to support the use of NSAIDs (25). NSAIDs inhibit COX-1 and COX-2 enzymes, and are classified as non-selective and selective inhibitors. NSAIDs inhibit COX, ultimately leading to a decreased production of AA and PG, thereby relieving pain from dysmenorrhea.
NSAIDs should be taken 1 to 2 days prior to the onset of menses and continued for 2 to 3 days. COX-2 inhibitors have been shown to be an effective treatment of dysmenorrhea, however, it has been linked to cardiac complications and is no longer recommended for treatment.
Tramadol may be used for pain as well. It is a centrally acting analgesic that acts by binding to the mu-opioid receptors and inhibiting the reuptake of norepinephrine and serotonin. It is not considered an addictive medication.
There is limited data on contraceptive methods for the treatment of dysmenorrhea, however they are commonly used for treatment. Combined hormonal contraceptives (CHCs), include the oral contraceptive pill (OCP), the transdermal patch and the vaginal ring. CHCs work by thinning the endometrial lining and inhibiting ovulation. Several studies have noted the effectiveness of CHCs in reducing dysmenorrhea in adolescents (26,27). Continuous use of the CHCs were found to provide more immediate relief of dysmenorrhea compared to using these methods in a cyclic fashion. Long-acting reversible contraceptive methods, such as the implant and the intrauterine device (IUD), were found to be of benefit in the treatment of dysmenorrhea. A few studies noted that adolescents rated a significant improvement in their symptoms with the use of the levonorgestrel-containing IUD and with the use of the etonogestrel implant. Endometrial thinning, which reduces the PG and LT production, is the proposed mechanism of action for these methods (28,29).
If the dysmenorrhea is relieved by the contraceptive method the medications should be continued for at least 6 months. If pain continues after a trial of these first-line medications, imaging studies and/or laparoscopy should be performed looking for other causes of dysmenorrhea.
Secondary dysmenorrhea
Secondary dysmenorrhea refers to painful menstruation associated with pelvic abnormalities or disease. Ten percent of adolescents may experience secondary dysmenorrhea. Secondary dysmenorrhea is more likely to be associated with chronic pelvic pain (CPP), midcycle pain, dyspareunia, and irregular menstrual bleeding. Secondary dysmenorrhea typically occurs 12 months after establishing a menstrual cycle. There are several etiologies for secondary dysmenorrhea with endometriosis being one of the most common etiologies.
Etiology
Endometriosis, often thought of as a disease process in older women, has been a focus of extensive research in adolescents. Endometriosis was found to be the most common pathologic cause of CPP in adolescents (30). In one study by Janssen et al. (30), endometriosis was identified in 60% of adolescents who underwent laparoscopy for CPP. Another study found that 30% to 40% of adolescent cases had advanced-stage endometriosis (31).
There are many proposed reasons for developing endometriosis. Sampson’s theory of retrograde menstruation proposes that there is a retrograde transport of pieces of endometrium through the fallopian tubes at the time of menstruation that leads to seeding of the peritoneal cavity (32). Meyer’s theory postulates that totipotent cells undergo metaplastic transformation into functioning endometrium (33). Halban’s theory states that there is a metastasis of endometrial cells through vascular or lymphatic spread (34). There is also a theory that suggests there is a deficiency in the cellular immunity that allows ectopic endometrial tissue to proliferate (35). A theory of environmental exposures has been implicated as a factor in the development of endometriosis, as well (36). All these theories help explain endometriosis. The most likely etiology of endometriosis is multifactorial and all proposed theories may contribute to the disease process.
Clinical presentation
Older women may present with a triad of symptoms; pelvic pain, dyspareunia and infertility. However, the clinical presentation of endometriosis in adolescents is dysmenorrhea, menorrhagia, abnormal/irregular uterine bleeding, and at least one gastrointestinal symptom, and at least one genitourinary symptom (37). Adolescents may experience acyclic pain. Pain that interferes with daily functioning, i.e., missing school or other activities, was noted to be caused by endometriosis in adolescents (38). Adolescents with pain who did not respond to initial treatment with NSAIDs and combined hormonal medications, were more likely to suffer from endometriosis. Adolescents have described their pain as achy, throbbing, and stabbing in nature. Adolescents may also experience bowel symptoms, such as constipation, dyschezia, and intestinal cramping. Sexually active adolescents may have dyspareunia. Some adolescents with endometriosis have reported symptoms of depression or anxiety (39).
Multiple risk factors are known for the development of endometriosis in the adolescent. These risk factors include, obstructive Müllerian anomalies, family history of endometriosis, school absences associated with menstruation, and early and prolonged use of oral contraceptive methods for the treatment of dysmenorrhea (40).
Other causes of secondary amenorrhea
Table 1 lists other potential causes of pelvic pain in adolescents. If endometriosis is less likely, one should consider possible Müllerian anomalies that cause obstruction of the outflow tract, i.e., imperforate hymen, transverse vaginal septum, or in rare cases obstructed hemivagina and ipsilateral renal anomaly (OHVIRA) syndrome. Usually, the uterus is found to be didelphys with obstructed hemivagina and agenesis of the ipsilateral kidney.
Table 1
| Ectopic pregnancy |
| Threatened or spontaneous abortion |
| Ovarian cyst/mass |
| Mumps oophoritis |
| Ovarian torsion |
| Hydrosalpinx |
| Endometritis (post therapeutic abortion, postpartum) |
| Pelvic inflammatory disease/Tubo-ovarian abscess |
| Vaginitis |
| Endometriosis |
| Adhesions |
| A history of sexual abuse |
| Gastrointestinal disorders |
| Urologic disorders |
Adnexal cysts can cause pain associated with menses. Many cysts are asymptomatic however, larger ovarian cysts may be associated with chronic pain that can be cyclical or noncyclical pain. Ultrasound or MRI would be necessary to detect these abnormalities. Depending on the anomaly either surgical or expectant management can be considered. Another etiology that may have similar features to dysmenorrhea is PID. Adolescents who are sexually active should be screened for STIs, such as gonorrhea and chlamydia.
Diagnosis
Diagnosis of endometriosis in the adolescent includes a thorough menstrual and related pain history. Questions should focus on the quality of the pain, timing, location and duration of the pain, and how the pain affects the adolescent’s life. Clinician may consider asking if the patient experiences pain during sex or defecation, if any family member has painful menses or a diagnosis of endometriosis, and having the patient describe how does the pain make them feel (41).
Pelvic exam may be considered in adolescents. A bimanual exam should be performed. Rectal exams can be an alternative to the pelvic exam in virginal adolescents (42). Of note in adult women, a mass or nodule may be palpated. However, in the adolescent, the pelvic exam may only elicit mild to moderate tenderness on exam. Imaging, such as CT, MRI, or ultrasound may not identify small lesions of endometriosis. A definitive diagnosis of endometriosis is made through laparoscopy and histopathologic confirmation.
Treatment
Figure 1 highlights a stepwise approach in evaluating and treating dysmenorrhea in adolescents, with high consideration of secondary causes, such as endometriosis. Failure of initial treatment of dysmenorrhea with NSAIDs and hormonal medications should prompt consideration of laparoscopic investigation. Outcome data on surgical treatment of endometriosis in adolescents is limited. There have been a few studies of adolescents which noted a reduction in pain after surgical excision of the endometriosis. In one study after excision, 73% of the adolescents treated were pain free or had greatly reduced pain (43). Another study noted that 23 months after surgical excision, adolescent patients had a statistically significant decrease in their pain and associated symptoms (44). Of note endometriosis due to obstructive anomalies have been reported to completely resolve after correction of the obstruction (45).
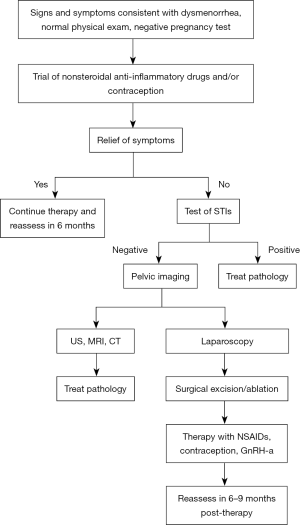
Recurrence of endometriosis in adolescents is more common than adults. Medical therapy after surgical excision should be initiated with the intention of reducing the chance for disease progression and pain control. The role of using hormonal medications is to suppress ovulation and growth of endometrial tissue. Endometriosis implants contain receptors for estrogen, progesterone, and androgens. Estrogens will stimulate the growth of the endometriosis tissue, and androgens cause atrophy of the tissues. Synthetic progestins inhibit endometrial growth through their androgenic properties. Because the endometrial tissue relies on steroid hormones for growth and function, the use of hormonal medication takes advantage of this physiology and suppresses tissue growth, thereby relieving pain.
CHCs methods such as the OCP, the transdermal patch and the vaginal ring have been shown to improve the pain of endometriosis by causing the endometrial implants to become inactive (46). A recent systematic review of the use of CHCs and progestin-only pills in the treatment of endometriosis in women found that both were associated with clinically significant reduction in non-cyclical pelvic pain and dyspareunia. Three of the studies reviewed found the use of CHCs reduced the risk of recurrence (47). Another systematic review of using continuous CHCs for the treatment of endometriosis in women found that post-operative continuous use of CHCs was associated with reduction in the recurrence of dysmenorrhea, delay in the presentation of dysmenorrhea, reduction in nonspecific pelvic pain, and the reduction in recurrence of endometrioma (48).
There are two progesterone only methods that are FDA approved for the treatment of endometriosis: norethindrone acetate (NETA) and depomedroxyprogesterone acetate (DMPA). Progesterone only methods should be considered for the patient that has contraindications to the use of estrogen. Several placebo-controlled trials showed the efficacy of progestins alone in alleviating the pain from endometriosis. DMPA and NETA were found to decrease the implant size on imaging and found to eliminate pelvic pain and dysmenorrhea in these patients (49). In addition, progestin therapies may be beneficial as second-line or adjunctive therapy (50).
Side effects should be considered when starting a progestin therapy. DMPA in particular, can cause weight gain, bloating, acne, headaches, emotional lability, and irregular menses. Long-term use of DMPA has been noted to cause loss of bone density in some patients and monitoring is advised (51).
The first-line and most widely used therapy for endometriosis is gonadotropin-releasing hormone agonists (GnRH-a). The continuous GnRH-a stimulation causes a down regulation of the pituitary, resulting in a hypoestrogenic state. A low estrogen state prevents bleeding in the implants and prevents additional seeding through retrograde menstruation (38). Several studies have investigated the effectiveness of GnRH-a in the suppression of endometriosis and pain relief, and found that GnRH-a improved the pain associated with endometriosis (52-54). A hypoestrogenic state can lead to side effects such as hot flashes, vaginal dryness, and decreased libido. Prolonged use of GnRH-a can cause a decrease in bone density as well. The FDA has approved the use of GnRH-a for only 6 consecutive months because of the concerns for decrease in bone density (55,56).
Several studies have examined the benefit of “add back” hormone therapy with GnRH-a therapy to decrease the side-effects of medication. These studies examined NETA and DMPA and the reduction of side effects when using GnRH-a. Studies have shown benefits to bone health when using a progestin with GnRH-a (57,58). A few randomized-controlled trials in adolescents with endometriosis, examined the effect of NETA with ethinyl estradiol (EE) versus NETA with placebo, and found that adolescents maintained bone health and had improved quality of life. NETA plus EE was superior to NETA alone for improving physical health quality of life (59,60).
For those patients who continue to have pain, NSAIDs have been effective in some cases. Naproxen sodium was shown to significantly improve endometriosis pain over placebo. However, many patients do not get relief from NSAIDs. Narcotics are not recommended for the treatment of dysmenorrhea and should be avoided. If pain persists, further evaluation and treatment is recommended (61,62).
Complementary and alternative medical therapies have been identified as possible treatments for dysmenorrhea due to endometriosis. The National Center for Complementary and Alternative Medicine did an extensive review of randomized control studies in reproductive age women (63). A few different herbal medications were promoted to alleviate symptoms of menstrual discomfort and premenstrual syndrome (PMS). Dong quai and primrose oil were both not found to be effective in treating dysmenorrhea and/or PMS (64,65). Diets low in fat and consumption of fish oil, were noted to decrease dysmenorrhea symptoms (66,67). Acupuncture has also been noted to provide benefit in treatment of dysmenorrhea and possibly endometriosis. Further studies are underway to evaluate its effectiveness (68).
The legalization of marijuana has opened the door to exploring its use as an alternative treatment for pain. Studies examining the use of marijuana for the treatment of pain associated with endometriosis have suggested that it may alleviate the pain because of its ability to interact with endocannabinoid receptors present in uterine muscle. One study of adolescents who received an antioxidant, transpolydatin and palmitoylethanolamine, a member of the cannabinoid family, found that the majority of adolescents treated had a decrease in their pelvic pain compared to placebo (69). However, there is insufficient evidence to support the safety of cannabinoid product use in adolescents with pelvic pain at this time.
Of note, there is no cure at this time for endometriosis. Treatment is aimed at suppressing the hormones. Endometriosis has been associated with infertility in women. Limiting progression of the disease may prevent anatomic distortion of the pelvic organs and fallopian tubes.
Conclusions
Dysmenorrhea is a common complaint in menstruating adolescent females. Menstrual pain is most often due to primary dysmenorrhea or functional pain and can affect the quality of life of adolescents. Some adolescents can experience secondary dysmenorrhea. First-line treatment for dysmenorrhea is NSAIDs and contraception. If the adolescent does not respond to first-line treatment evaluation for other causes should be considered. Mullerian anomalies, ovarian cysts and PID may be identified as the cause of secondary dysmenorrhea. Endometriosis should be considered as well. Early identification and treatment of endometriosis can preserve fertility and suppress the natural disease progression.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Maria Demma Cabral and Dilip R. Patel) for the series “Adolescent Gynecology” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.06.09). The sereis "Adolescent Gynecology" was commissioned by the editorial office without any funding or sponsorship. The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harlow SD, Campbell OM. Epidemiology of menstrual disorders in developing countries: a systematic review. BJOG 2004;111:6-16. [Crossref] [PubMed]
- Weissman AM, Hartz AJ, Hansen MD, et al. The natural history of primary dysmenorrhoea: a longitudinal study. BJOG 2004;111:345-52. [Crossref] [PubMed]
- De Sanctis V, Soliman A, Bernasconi S, et al. Primary Dysmenorrhea in adolescents: Prevalence, impact and recent knowledge. Pediatr Endocrinol Rev 2015;13:512-20. [PubMed]
- Parker MA, Sneddon AE, Arbon P. The menstrual disorder of teenagers (MDOT) study: determining typical menstrual patterns and menstrual disturbance in a large population-based study of Australian teenagers. BJOG 2010;117:185-92. [Crossref] [PubMed]
- Zannoni L, Giorgi M, Spagnolo E, et al. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J Pediatr Adolesc Gynecol 2014;27:258-65. [Crossref] [PubMed]
- Hailemeskel S, Demissie A, Assefa N. Primary dysmenorrhea magnitude, associated risk factors, and its effect on academic performance: evidence from female university students in Ethiopia. Int J Womens Health 2016;8:489-96. [Crossref] [PubMed]
- Baker FC, Driver HS, Rogers GG, et al. High nocturnal body temperatures and disturbed sleep in women with primary dysmenorrhea. Am J Physiol 1999;277:E1013-21. [PubMed]
- Dorn LD, Negriff S, Huang B, et al. Menstrual symptoms in adolescent girls: association with smoking, depressive symptoms, and anxiety. J Adolesc Health 2009;44:237-43. [Crossref] [PubMed]
- Chan WY, Hill JC. Determination of menstrual prostaglandin levels in nondysmenorrheic and dysmenorrhic subjects. Prostaglandins 1978;15:365-75. [Crossref] [PubMed]
- Rees MC, Anderson AB, Demers M, et al. Prostaglandins in menstrual fluid in menorrhagia and dysmenorrhea. Br J Obstet Gynaecol 1984;91:673-80. [Crossref] [PubMed]
- Rees MC, DiMarzo V, Tippins JR, et al. Leukotriene release by endometrium and myometrium throughout the menstrual cycle in dysmenorrhea and menorrhagia. J Endocrinol 1987;113:291-295. [Crossref] [PubMed]
- Harel Z, Lilly C, Riggs S, et al. Urinary leukotriene (LT) E(4) in adolescents with dysmenorrhea. J Adolesc Health 2000;27:151-4. [Crossref] [PubMed]
- Andersch B, Milsom I. An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol 1982;144:655-60. [Crossref] [PubMed]
- Balbi C, Musone R, Menditto A, et al. Influence of menstrual factors and dietary habits on menstrual pain in adolescence age. Eur J Obstet Gynecol Reprod Biol 2000;91:143-8. [Crossref] [PubMed]
- Patel V, Tanksale V, Sahasrabhojanee M, et al. The burden and determinants of dysmenorrhea: a population-based survey of 2262 women in Goa, India. BJOG 2006;113:453-63. [Crossref] [PubMed]
- Burnett MA, Antao V, Black A, et al. Prevalence of primary dysmenorrhea in Canada. J Obstet Gynaecol Can 2005;27:765-70. [Crossref] [PubMed]
- Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev 2014;36:104-13. [Crossref] [PubMed]
- Unsal A, Tozun M, Aslan G, et al. Evaluation of dysmenorrhea among women and its impact on quality of life in a region of Western Turkey. Pak J Med Sci 2010;26:142-7.
- Wang L, Wang X, Wang W, et al. Stress and dysmenorrhea: a population-based prospective study. Occup Environ Med 2004;61:1021-6. [Crossref] [PubMed]
- Nohara M, Momoeda M, Kubota T, et al. Menstrual cycle and menstrual pain problems and related risk factors among Japanese female workers. Ind Health 2011;49:228-34. [Crossref] [PubMed]
- Tavallaee M, Joffres MR, Corber SJ, et al. The prevalence of menstrual pain and associated risk factors among Iranian women. J Obstet Gynaecol Res 2011;37:442-51. [Crossref] [PubMed]
- Pitts MK, Ferris JA, Smith AM, et al. Prevalence and correlates of three types of pelvic pain in a nationally representative sample of Australian women. Med J Aust 2008;189:138-43. [PubMed]
- Ohde S, Tokuda Y, Takahashi O, et al. Dysmenorrhea among Japanese women. Int J Gynaecol Obstet 2008;100:13-7. [Crossref] [PubMed]
- László KD, Gyorffy Z, Adam S, et al. Work-related stress factors and menstrual pain: a nation-wide representative survey. J Psychosom Obstet Gynaecol 2008;29:133-8. [Crossref] [PubMed]
- Marjoribanks J, Proctor M, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev 2010;CD001751 [PubMed]
- Davis AR, Westhoff C, O’Connell K, et al. Oral contraceptives for dysmenorrhea in adolescent girls: a randomized clinical trial. Obstet Gynecol 2005;106:97-104. [Crossref] [PubMed]
- Dmitrovic R, Kunselman AR, Legro RS. Continuous compared with cyclic oral contraceptives for the treatment of primary dysmenorrhea. A randomized controlled trial. Obstet Gynecol 2012;119:1143-50. [Crossref] [PubMed]
- Aslam N, Blunt S, Latthe P. Effectiveness and tolerability of levonorgestrel intrauterine system in adolescents. J Obstet Gynaecol 2010;30:489-91. [Crossref] [PubMed]
- Suhonen S, Haukkamaa M, Jakobsson T, et al. Clinical performance of a levonorgestrel-releasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception 2004;69:407-12. [Crossref] [PubMed]
- Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update 2013;19:570-82. [Crossref] [PubMed]
- Smorgick N, As-Sanie S, Marsh CA, et al. Advanced stage endometriosis in adolescents and young women. J Pediatr Adolesc Gynecol 2014;27:320-3. [Crossref] [PubMed]
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of the endometrial tissue into the peritoneal cavity. AM J Obstet Gynecol 1927;14:422-69. [Crossref]
- Laufer MR, Goldstein DP. Gynecologic Pain: Dysmenorrhea, acute and chronic pelvic pain, endometriosis, and premenstrual syndrome. In: Emans JS, Laufer MR, Goldstein DP. editors. Pediatric and Adolescent Gynecology. Philadelphia: Lippincott Williams and Wilkins, 2005:440.
- Bulun SE, Yilmaz BD, Sison C, et al. Endometriosis. Endocr Rev 2019. [Epub ahead of print].
- Halme J, Becker S, Haskill S. Altered maturation and function of peritoneal macrophages: possible role in pathogenesis of endometriosis. Am J Obstet Gynecol 1987;156:783-9. [Crossref] [PubMed]
- Rier S, Foster WG. Environmental dioxins and endometriosis. Toxicol Sci 2002;70:161-70. [Crossref] [PubMed]
- Dun EC, Kho KA, Morozov VV, et al. Endometriosis in adolescents. JSLS 2015;19:e2015.00019.
- Laufer MR, Sanfilippo J, Rose G. Adolescent endometriosis: diagnosis and treatment approaches. J Pediatr Adolesc Gynecol 2003;16:S3-11. [Crossref] [PubMed]
- Smorgick N, Marsh CA, As-Sanie S, et al. Prevalence of pain syndromes, mood conditions, and asthma in adolescents and young women with endometriosis. J Pediatr Adolesc Gynecol 2013;26:171-5. [Crossref] [PubMed]
- Templeman C. Adolescent endometriosis. Curr Opin Obstet Gynecol 2012;24:288-92. [Crossref] [PubMed]
- Steenberg CK, Tanbo TG, Qvigstad E. Endometriosis in adolescence: predictive markers and management. Acta Obstet Gynecol Scand 2013;92:491-5. [Crossref] [PubMed]
- Sarıdoğan E. Endometriosis in teenagers. Womens Health (Lond) 2015;11:705-9. [Crossref] [PubMed]
- Stavroulis AI, Saridogan E, Creighton SM, et al. Laparoscopic treatment of endometriosis in teenagers. Eur J Obstet Gynecol Reprod Biol 2006;125:248-50. [Crossref] [PubMed]
- Yeung P Jr, Sinervo K, Winer W, et al. Complete laparoscopic excision of endometriosis in teenagers: is postoperative hormonal suppression necessary? Fertil Steril 2011;95:1909-12. [Crossref] [PubMed]
- Sanfilippo JS, Wakim NG, Schikler KN, et al. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol 1986;154:39-43. [Crossref] [PubMed]
- Kistner RW. The treatment of endometriosis by inducing pseudopregnancy with ovarian hormones: a report of 58 cases. Fertil Steril 1959;10:539-56. [Crossref]
- Grandi G, Barra F, Ferrero S, et al. Hormonal contraception in women with endometriosis: a systematic review. Eur J Contracept Reprod Health Care 2019;24:61-70. [Crossref] [PubMed]
- Zorbas KA, Economopoulos KP, Vlahos NF. Continuous versus cyclic oral contraceptives for the treatment of endometriosis: A systematic review. Arch Gynecol Obstet 2015;292:37-43. [Crossref] [PubMed]
- Moghissi KS, Boyce CR. Management of endometriosis with oral medroxyprogesterone. Obstet Gynecol 1976;47:265-7. [PubMed]
- Cosson M, Querleu D, Donnez J, et al. Dienogest is as effective as triptorelin in the treatment of endometriosis after laparoscopic surgery: results of a prospective, multicenter, randomized study. Fertil Steril 2002;77:684-92. [Crossref] [PubMed]
- Cromer BA, Blair JM, Mahan JD, et al. A prospective comparison of bone density in adolescent girls receiving depo-medroxyprogesterone acetate (Depo-Provera), levonorgestrel (Norplant), or oral contraceptives. J Pediatr 1996;129:671-6. [Crossref] [PubMed]
- Burry KA. Nafarelin in the management of endometriosis: quality of life assessment. Am J Obstet Gynecol 1992;166:735-9. [Crossref] [PubMed]
- Miller JD. Quantification of endometriosis-associated pain and quality of life during the stimulatory phase of gonadotropin-releasing hormone agonist therapy: a double-blind, randomized, placebo-controlled trial. Am J Obstet Gynecol 2000;182:1483-8. [Crossref] [PubMed]
- Henzl MR, Corson SL, Moghissi K, et al. Administration of nasal narafelin as compared with oral danazol for endometriosis. N Engl J Med 1988;318:485-9. [Crossref] [PubMed]
- Dawood MY, Lewis V, Ramos J. Cortical and trabecular bone mineral content in women with endometriosis: effect of gonadotropin releasing hormone agonist and danazol. Fertil Steril 1989;52:21-6. [Crossref] [PubMed]
- Fogelman I. Gonadotropin-releasing hormone agonists and the skeleton. Fertil Steril 1992;57:715-24. [Crossref] [PubMed]
- Surrey ES, Gambone JC, Lu JK, et al. The effects of combining norethindrone with a gonadotropin-releasing hormone agonist in the treatment of symptomatic endometriosis. Fertil Steril. 1990;53:620-6. [Crossref] [PubMed]
- Cedars MI, Lu JK, Meldrum DR, et al. Treatment of endometriosis with a long-acting gonadotropin-releasing hormone agonist plus medroxyprogesterone acetate. Obstet Gynecol 1990;75:641-5. [PubMed]
- DiVasta AD, Feldman HA, Sadler Gallagher J, et al. Hormonal add-back therapy for females treated with gonadotropin-releasing hormone agonist for endometriosis: a randomized controlled trial. Obstet Gynecol 2015;126:617-27. [Crossref] [PubMed]
- Bedaiwy MA, Allaire C, Alfaraj S. Long-term medical management of endometriosis with dienogest and with a gonadotropin-releasing hormone agonist and add-back therapy. Fertil Steril 2017;107:537-48. [Crossref] [PubMed]
- Smith RP. Cyclic pelvic pain and dysmenorrhea. Obstet Gynecol Clin North Am 1993;20:753-64. [PubMed]
- Brown J, Crawford TJ, Allen C, et al. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev 2017;1:CD004753 [PubMed]
- Fugh-Berman A, Kronenberg F. Complementary and alternative medicine (CAM) in reproductive-age women: a review of randomized controlled trials. Reprod Toxicol 2003;17:137-52. [Crossref] [PubMed]
- Hirata JD, Swiersz LM, Zell LM, et al. Does dong quai have estrogenic effects in postmenopausal women? A double blind, placebo-controlled trial. Fertil Steril 1997;68:981-6. [Crossref] [PubMed]
- Khoo SK, Munro C, Battistutta D. Evening primrose oil and treatment of premenstrual syndrome. Med J Aust 1990;153:189-92. [PubMed]
- Barnard ND, Scialli AR, Hurlock D, et al. Diet and sex-hormone binding globulin, dysmenorrhea, and premenstrual symptoms. Obstet Gynecol 2000;95:245-50. [PubMed]
- Harel Z, Biro FM, Kottenham RK, et al. Supplementation with omega-3 polyunsaturated fatty acids in the management of dysmenorrhea in adolescents. Am J Obstet Gynecol 1996;174:1335-8. [Crossref] [PubMed]
- Helms JM. Acupuncture for management of primary dysmenorrhea. Obstet Gynecol 1987;69:51-6. [PubMed]
- Tartaglia E, Armentano M, Giugliano B, et al. Effectiveness of the association N-Palmitoylethanolamine and Transpolydatin in the treatment of primary dysmenorrhea. J Pediatr Adolesc Gynecol 2015;28:447-50. [Crossref] [PubMed]
Cite this article as: Dharmapuri S. Dysmenorrhea in adolescents. Pediatr Med 2019;2:34.

