
Acute pelvic inflammatory disease: a narrative review
Introduction
Pelvic inflammatory disease (PID) is a major sexually transmitted disease typically involving sexually active adolescent and young adult females (1). Its presentation can be confusing with an extensive differential diagnosis. This discussion considers principles of PID management based on guidelines from the United States Centers for Disease Control and Prevention (U.S. CDC) and the World Health Organization (WHO). Specific factors need specific consideration in the management of PID; these include the presence of human immunodeficiency virus infection, use of intrauterine devices (IUDs), incarceration, and sexual violence.
Methods
An electronic search was performed on MEDLINE to identify relevant articles from 2010 to 2018.
Definition
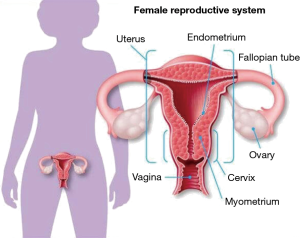
PID is generally a sexually transmitted disease characterized by upper genital tract infection in females (1) (Figure 1). It is often initiated by the presence of Neisseria gonorrhoeae (N. gonorrhoeae) and/or Chlamydia trachomatis (C. trachomatis) in the lower genital tract that ascend to infect the uterus, fallopian tubes and ovaries. PID often becomes a polymicrobial infection due to the presence of various vaginal-cervical endogenous microbes (1,2).
Epidemiology
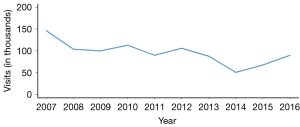
There are approximately one million PID cases that are identified each year in the U.S. and approximately one-third are diagnosed in adolescent females. After a steady decline in initial office visit for PID until 2014, a rising trend is recently reported (Figure 2). Finding precise PID epidemiologic figures can be challenging due to differences in surveillance in various regions as well as countries, natural variation in PID prevalence, and difficulty of distinguishing cervicitis from overt PID (1,3,4).
Pathophysiology
Classic PID is an infection that begins in the cervical-vaginal region and ascends to the upper genital tract resulting in a combination of features; these include acute salpingitis, perihepatitis, endometritis, oophoritis, pelvic peritonitis and/or tubo-ovarian abscess (TOA) (1,2,5). Scarring, adhesions and obstruction of the fallopian tubes may result from PID-induced inflammation. Loss of the ciliary epithelial cells of the fallopian tube impairs ovum transport and augments the risk for infertility as well as ectopic pregnancy; chronic pelvic pain may develop due to adhesions (6).
Studies reveal that C. trachomatis-induced PID is more frequent in the 16- to 24-year old female [versus Neisseria gonorrhoeae (N. gonorrhoeae) initiation] (3). Microbiology findings of PID research show that C. trachomatis is found in up to 43% (10–43%) of PID cases and N. gonorrhoeae is noted in up to 50% (25–50%). Other microbes are seen in 30% of PID cases; these include enteric and respiratory microbes, cervical pathogens (i.e., Mycoplasma genitalium), bacterial vaginosis agents, and other bacteria (i.e., anaerobic and facultative) (Table 1).
Table 1
| Chlamydia trachomatis |
| Neisseria gonorrhoeae |
| Gardnerella vaginalis |
| Haemophilus influenzae |
| Bacteroides species (B. fragilis, bivius, disiens) |
| Mycoplasma genitalium |
| Group B streptococcus (S. agalactiae) |
| Coliforms (Enterobacteriaceae) |
| Peptostreptococcus |
| Streptococcus faecalis |
| Ureaplasma urealyticum |
| Neisseria meningitides |
| Mycoplasma hominis |
| Enterococcus |
| Cytomegalovirus |
| Other anaerobes |
Risk factors
Research has identified a number of risk factors in PID development that include young age (i.e., adolescence or young adulthood), ectropion of young adolescent females, immature immune system, multiple coital partners, ineffective condom usage, past PID, presence of bacterial vaginosis, vaginal douching, coitus during menstruation, and history of non-barrier contraception (1).
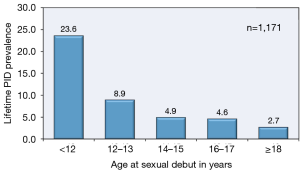
The highest PID prevalence is found in adolescent females 15 to 19 years of age who initiate their coital experience early in adolescence (Figure 3), have multiple coital partners, and fail to utilize effective contraception or contraceptive methods in a recommenced manner (1,7). An additional risk factor for young adolescent females is the presence of immature cervix that contains columnar epithelium transitional zone that can be a positive milieu for N. gonorrhoeae and C. trachomatis.
Trauma to the endocervical canal from an IUD may facilitate the ascent of microorganisms into the endometrial cavity. Multifilament IUD tails strings were implicated earlier with increased risk for infection; however, current research reveals that PID risks do not rise with insertion of monofilament tail string IUDs (1,8). Even so, expert recommendations for the placement of IUDs in adolescent females usually state that C. trachomatis and N. gonorrhoeae screening occur when inserting the IUD (9,10).
Complicating the PID in the adolescent females is their immature immune system; this includes the immaturity of antibody and CD4+ T cell responses that fail to eliminate C. trachomatis and the resultant potential for a silent infection (11). Chronic fallopian tube damage can result from chronic inflammation due to various factors that include cell-autonomous immunity and the effects of interstitial or stromal cells (i.e., telocytes) (Table 2) (1,12-14).
Table 2
| Stimulation of the innate immune receptor with Toll-like receptor 1 |
| Anti-C trachomatis antibodies (i.e., 60-kDa heat shock protein 60) |
| CD4+ T cell responses |
| Immune responses of TH1 |
| Immune responses of TH17 |
C. trachomatis, Chlamydia trachomatis.
Not only can repeated C. trachomatis infection lead to fallopian tube damage, but tubal injury may occur from N. gonorrhoeae via release of lytic transglycosylases (LTg) (1). LTgA and LTgD can induce the development and release of peptidoglycans that can damage fallopian tube cells (15,16).
Specific considerations
Sexual violence
Individuals may be hesitant to get medical care if they have been victims of sexual violence including rape (17). Medical practitioners should understand that these adolescents and young adults may be reluctant to seek timely medical care if there is a history of sexual violence. Psychological support is needed by caring and understanding health care professionals to provide beneficial support in such potentially difficult circumstances.
Incarceration
Adolescents in jail or jail-like facilities have an increased incidence of C. trachomatis, N. gonorrhoeae, PID and other sexually transmitted infections (STIs) (1). Thus, it is important to provide robust STI screening programs to these youths with appropriate treatment as well (18).
Lesbian, bisexual, and transgender youth
Other youth who may be reluctant to seek healthcare for their sexuality issues include those who identify as lesbian, bisexual and transgender; also, medical practitioners may assume that some of these persons are not at risk for PID (19,20). Thus, medical practitioners caring for all youth must be vigilant for the health care needs of all their young patients and obtain a careful as well as detailed history on their medical needs (21).
HIV
Females diagnosed with PID should be screened for other STIs including HIV and also for being victims of sexual violence (22,23). Management of females with PID in women with HIV is similar to those without associated HIV; however, some data suggests that this combination of disorders may induce an increase in concomitant infection with certain microbes (i.e., streptococci, Mycoplasma hominis) as well as increased risk for TOA (24).
Clinical features
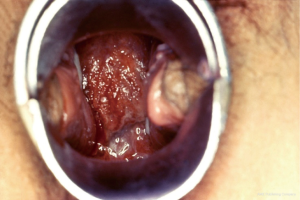
A wide variety of symptoms can be seen in females with PID and thus, accurate diagnosis can be clinically challenging (1). Patients may exhibit few or no symptoms, whereas others have acute, serious illness. The most common presenting complaint is lower abdominal pain. Gonorrheal PID is the most severe form whereas chlamydial PID is more likely to be subclinical with little or no symptoms, but with potentially adverse long-term consequences (6). In a sample of endometrial biopsies, 13% females with PID were diagnosed with subclinical PID (25,26). Although sparse symptoms may be seen in PID, its classic presentation is vaginal (cervical) discharge (Figure 4) and lower abdominal (lower quadrant; pelvic) pain (1).
Other features of PID include vaginal bleeding, urinary symptoms (i.e., dysuria, urinary frequency), post-coital bleeding and/or dyspareunia (1-3,5). Others include fever, chills, and protean gastrointestinal symptoms (i.e., nausea, emesis, constipation, and diarrhea). The condition can also manifest with an acute abdominal crisis with rebound tenderness and reduced bowel sounds. A pelvic examination may reveal cervical motion tenderness and there may or may not be uterine or adnexal tenderness (1-3,5).
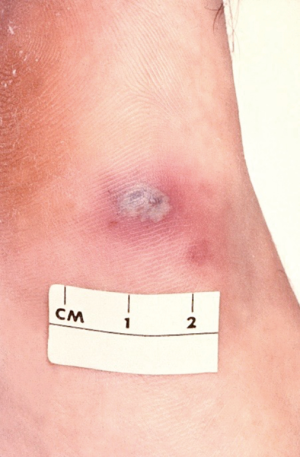
Studies reveal that about 4% (3–10%) of those with PID also have Fitz-Hugh-Curtis syndrome (perihepatitis) due to the spread of the lower genital tract inflammation/infection in the paracolic gutter that induces inflammation of the liver’s Glisson’s capsule and local peritoneum (1). Perihepatitis symptomatology includes fever, nausea, emesis, abdominal pain (right upper quadrant pain), right pleural effusion and/or right shoulder pain (1,27-31). In such cases there is typically right upper quadrant tenderness but no lower abdominal pain. Medical practitioners should consider perihepatitis (Fitz-Hugh-Curtis syndrome) in sexually active females who have right upper quadrant pain (32). Severe infection with N. gonorrhoeae may progress to disseminated gonococcal infection (Figure 5).
Diagnosis
Diagnostic criteria for PID from the U.S. CDC are listed in Table 3 that include minimal criteria, additional criteria and specific criteria (24) (Figure 6). All females suspected of PID should be tested for N. gonorrhoeae and C. trachomatis infection using nucleic acid amplification tests. Although the diagnosis of PID in most cases is based on clinical findings, when indicated, it can be aided by the following: laparoscopy (81% sensitivity versus 100% specificity), magnetic resonance imaging, transvaginal sonography (30% sensitivity versus 67% specificity), and endometrial biopsy (74% sensitivity versus 84% specificity) (1,24,32).
Table 3
| Category of criteria | Specific criteria data |
|---|---|
| Minimal criteria (≥1) | Cervical motion tenderness, or uterine tenderness, or adnexal tenderness |
| Amdditional criteria (≥1 to support minimal criteria for PID) | Oral temperature over 101 °F (over 38.3 °C) |
| Abnormal cervical mucopurulent discharge or cervical friability | |
| Presence of abundant numbers of WBC on saline microscopy of vaginal fluid | |
| Elevated erythrocyte sedimentation rate | |
| Elevated C-reactive protein; and | |
| Laboratory documentation of cervical infection with N. gonorrhoeae or C. trachomatis | |
| Most specific criteria | Endometrial biopsy with histopathological evidence of endometritis |
| Findings of PID on laparoscopy | |
| Ultrasound (transvaginal) or magnetic resonance imaging showing fallopian tubes that are thick and filled with fluid; may be free fluid in the pelvis or a tubo-ovarian complex; or | |
| Doppler studies suggestive of pelvic infection via tubal hyperemia |
U.S. CDC, United States Centers for Disease Control and Prevention; PID, pelvic inflammatory disease.
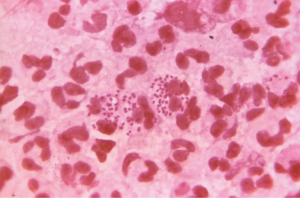
The diagnosis of PID in emergency rooms and clinics is often based on clinical criteria, with or without additional laboratory and imaging tests (33). Clinical findings have a sensitivity of 87% and a specificity of 50% versus 83% sensitivity and 26% specificity of endometrial culture (1,2,24,32).
Table 4 lists the differential diagnosis of PID and reveals the clinical challenge that can arise in a sexually active female with PID-like symptoms. Many possibilities must be considered including genitourologic, gynecologic, gastrointestinal, rheumatologic, hematologic and others (1,24,32). A pregnancy test should be performed to exclude the possibility of ectopic pregnancy. An augmented level of serum procalcitonin can be a marker for TOA (34). Medical practitioners must also consider that PID can develop in non-sexually active females in uncommon cases (35-38). In virginal adolescents, PID may originate from the lower genital tract, urinary tract, gastrointestinal tract and may be associated with genital or other anomalies (39).
Table 4
| Acute intermittent porphyria |
| Adnexal torsion |
| Appendicitis |
| Constipation |
| Cystitis (urinary tract infection) |
| Diverticulitis |
| Dysmenorrhea |
| Endometriosis |
| Ectopic pregnancy |
| Fallopian tube torsion |
| Functional abdominal pain |
| Gastroenteritis (as due to Yersinia enterocolitica or Campylobacter fetus) |
| Genital trauma |
| Henoch-Schonlein syndrome |
| Hemolytic-uremic syndrome |
| Inflammatory bowel disease |
| Irritable bowel disease |
| Lead intoxication |
| Lupus serositis |
| Meckel’s diverticulum |
| Mesenteric lymphadenitis |
| Mesenteric vascular disease |
| Ovarian cyst (with or without torsion or rupture) |
| Ovarian neoplasm (including teratoma rupture) |
| Ovulation (Mittelschmerz) |
| Pelvic adhesions |
| Pregnancy complication |
| Pyelonephritis |
| Reiter’s syndrome |
| Septic abortion |
| Sickle cell crisis |
| Urethritis |
| Ureterocele |
| Urolithiasis |
Treatment
Treatment of PID involves an early diagnosis along with the recognized protocols such as the 2001 WHO (40) (Table 5) or the 2015 CDC sexually transmitted diseases guidelines (Table 6) (24). Such antibiotic courses cover various microbes seen in PID such as C. trachomatis, N. gonorrhoeae, Mycoplasma genitalium, and various facultative/anaerobic bacteria (1,24). Use of these recommended antibiotics should lead to observable symptomatology improvement in two to three days that includes no fever, reduction in abdominal pain (tenderness, rebound), and reduced pelvic pain (less cervico-utero-adnexal motion tenderness). Table 7 lists indications for hospitalization.
Table 5
| Type of patient | Recommended antibiotics |
|---|---|
| Ambulatory patients | Ceftriaxone 250 mg IM in a single dose followed by doxycycline 100 mg orally every 12 hours for 10 days (contraindicated during pregnancy), plus metronidazole 400–500 mg orally every 8 hours for 10 days (contraindicated during pregnancy) |
| Hospitalized patients with moderate or severe disease | Ceftriaxone 25 mg IM every 12 hours for at least 4 days (or for 48 hours after clinical improvement occurs), followed by doxycycline 100 mg orally every 12 hours for 10–14 days (contraindicated during pregnancy) |
| Hospitalized patients with very severe disease | Gentamicin 5–7 mg/kg IV or IM every 24 hours or 1.5–2.0 mg/kg IV or IM every 8 hours for at least 4 days (or for 48 hours after clinical improvement occurs; contraindicated during pregnancy; plus clindamycin 900 mg IV every 8 hours for at least 4 days (for 48 hours after clinical improvement occurs) followed by doxycycline 100 mg orally every 12 hours for 10–14 days (contraindicated during pregnancy) |
PID, pelvic inflammatory disease; IM, intramuscular; IV, intravenous.
Table 6
| Method of delivery | Recommended antibiotics |
|---|---|
| Oral/intramuscular | Ceftriaxone 250 mg IM in a single dose or cefoxitin 2 g IM in a single dose and probenecid 1 g orally concurrently or other parenteral third generation cephalosporin (as ceftizoxime or cefotaxime); plus doxycycline 100 mg orally twice a day for 14 days with or without metronidazole 500 mg orally twice a day for 14 days |
| Parenteral | Regimen A: cefotetan 2 g IV every 12 hours or cefoxitin 2 g IV every 6 hours plus doxycycline 100 mg orally or IV every 12 hours |
| Regimen B: clindamycin 900 mg IV every 8 hours plus gentamicin loading dose IV or IM (2 mg/kg body weight) followed by a maintenance dose (1.5 mg/kg) every 8 hours. Single daily dosing (3–5 mg/kg) can be substituted | |
| Alternative: ampicillin/Sulbactam 3 g IV every 6 hours plus doxycycline 100 mg orally or IV every 12 hours |
U.S. CDC, United States Centers for Disease Control and Prevention; STI, sexually transmitted infection; PID, pelvic inflammatory disease; IM, intramuscular; IV, intravenous.
Table 7
| High fever |
| Intractable nausea or vomiting (including loss of medications due to vomiting) |
| Inability to follow outpatient protocols |
| Pregnancy |
| Possibility of appendicitis or other surgical emergency |
| Tubo-ovarian abscess |
| Failure of oral antimicrobial therapy |
PID, pelvic inflammatory disease.
Intravenous antibiotics can be converted to oral administration after 24 hours of improvement. The use of metronidazole can also treat bacterial vaginosis that may also be present (1). Oral use of doxycycline is preferred since intravenous administration of this antibiotic can be quite painful (1).
A variety of problems arise in treatment including increasing bacterial resistance to antibiotics, i.e., quinolone-resistant N. gonorrhoeae (QRNG) and reduced efficacy of third-generation cephalosporins (1,41). Therefore, quinolones are no longer recommended for the treatment of gonorrhoeae (41,42). The ease of travel across regions and countries has led to continuous changes in other bacteria as well—such as the prevalence and antibiotic resistances of Mycoplasma genitalium, Ureaplasma urealyticum and others (43-45). Complicating this picture is the failure of some medical practitioners to follow established guidelines (46-49).
Careful management of PID is important to reduce discomfort, improve symptoms as soon as possible, and potentially reduce the complications that include chronic pelvic pain, ectopic pregnancy and infertility (1,26,50). In the classic research of Weström et al. dealing with a large cohort of women, laparoscopic evaluations revealed a PID-associated infertility rate of 16% (versus 2.7% in controls); also, 9.1% of post-PID pregnancy were ectopic pregnancies (versus 1.4% of controls) (50).
According to the WHO (51) and CDC (52) Medical Eligibility Criteria for Contraceptive Use, there is insufficient evidence to recommend removal of the IUD in the case of acute PID. However, close clinical follow-up is recommended if the IUD is left in place (53). Patients with PID should be tested for other STIs (including HIV). Sexual partner/s should be evaluated and treated when indicated. The U.S. CDC recommends treatment of the PID patient’s sexual partner (s) over the past 2 months even though they may be asymptomatic; principles of expedited partner therapy (EPT) are suggested (1,24). The treated PID patient should be seen in 3 to 6 months post-treatment and provided with further sexuality education (1,24).
Prevention
Various STI/PID screening programs have been utilized around the world over the past few decades such as that of the annual C. trachomatis screening recommended by the U.S. Preventive Service Task Force (USPSTF) for all sexually active females under age 26 years of age (54). Medical practitioners can also recommend screening utilizing the “self-taken swab” that is done by the person being screened in which the examination of the swab can detect both C. trachomatis and N. gonorrhoeae with nucleic acid amplification technology (55,56). One study noted a 56% lowering of PID incidence in sexually active females (18 to 34 years of age) who received appropriate C. trachomatis screening (57). Limitations to such success of screening programs may occur if the screening is limited, medical practitioners are not motivated to provide/encourage such policies, or public health officials in various countries view such programs as too costly (58-60). Table 8 lists other prevention considerations that have been utilized to reduce PID as well as other STIs.
Table 8
| Education to delay adolescent coital activity |
| Education about correct condom use |
| Education of potential cause of abdominal pain and need for medical evaluation in such situations |
| Comprehensive sexuality education in schools |
| Include sexuality education to those at high-risk for STIs—runaway teens, incarcerated teens |
| Further research on immunological defenses for infections |
| Further research on vaccines for STIs (as C. trachomatis and N. gonorrhoeae) |
STI, sexually transmitted infection; C. trachomatis, Chlamydia trachomatis; N. gonorrhoeae, Neisseria gonorrhoeae.
Conclusions
PID is a common sexually transmitted disease found among sexually active adolescents and young adults as well as older adult females (1). Those with PID may present with vaginal-cervical discharge, lower abdominal pain, cervical motion tenderness and bilateral adnexal tenderness. A variety of symptomatology may occur including a paucity of symptoms. The differential diagnosis is considerable and a careful evaluation is needed to identify the correct diagnosis. PID requires antibiotics as per established protocols such as found with the CDC and the WHO. Careful follow-up is needed to reduce potential PID complications. Each country should establish comprehensive sexuality education that includes STI prevention education (1,61).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Maria Demma Cabral and Dilip R. Patel) for the series “Adolescent Gynecology” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.07.05). The series “Adolescent Gynecology” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Greydanus DE, Dodich C. Pelvic inflammatory disease: a poignant, perplexing, potentially preventable problem for patients and physicians. Curr Opin Pediatr 2015;27:92-9. [Crossref] [PubMed]
- Ford GW, Decker CF. Pelvic inflammatory disease. Dis Mon 2016;62:301-5. [Crossref] [PubMed]
- Scholes D, Satterwhite CL, Yu O, et al. Long-term trends in Chlamydia trachomatis infections and related outcomes in a U.S. managed care population. Sex Transm Dis 2012;39:81-8. [Crossref] [PubMed]
- Datta SD, Torrone E, Kruszon-Moran D, et al. Chlamydia trachomatis trends in the United States among persons 14 to 39 years of age, 1999-2008. Sex Transm Dis 2012;39:92-6. [Crossref] [PubMed]
- Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. N Engl J Med 2015;372:2039-48. [Crossref] [PubMed]
- Jennings LK, Krywko DM. Pelvic Inflammatory Disease (PID). StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2018.
- Simms I, Stephenson JM, Mallinson H, et al. Risk factors associated with pelvic inflammatory disease. Sex Transm Infect 2006;82:452-7. [Crossref] [PubMed]
- Lee NC, Rubin GL, Borucki R. The intrauterine device and pelvic inflammatory disease revisited: new results from the Women's Health Study. Obstet Gynecol 1988;72:1-6. [PubMed]
- Sufrin CB, Postlethwaite D, Armstrong MA, et al. Neisseria gonorrhea and Chlamydia trachomatis screening at intrauterine device insertion and pelvic inflammatory disease. Obstet Gynecol 2012;120:1314-21. [Crossref] [PubMed]
- Caddy S, Yudin MH, Hakim J, et al. Best practice to minimize risk of infection with intrauterine device insertion. J Obstet Gynaecol Can 2014;36:266-74. [Crossref] [PubMed]
- Darville T. Recognition and treatment of chlamydial infections from birth to adolescence. Adv Exp Med Biol 2013;764:109-22. [Crossref] [PubMed]
- Vicetti Miguel RD, Quispe Calla NE, Pavelko SD, et al. Intravaginal Chlamydia trachomatis challenge infection elicits TH1 and Th17 immune responses in mice that promote pathogen clearance and genital tract damage. PLoS One 2016;11:e0162445. [Crossref] [PubMed]
- Taylor BD, Zheng X, Darville T, et al. Whole-exome sequencing to identify novel biological pathways associated with infertility after pelvic inflammatory disease. Sex Transm Dis 2017;44:35-41. [Crossref] [PubMed]
- Yang XJ. Telocytes in inflammatory gynaecologic diseases and infertility. Adv Exp Med Biol 2016;913:263-85. [Crossref] [PubMed]
- Chan YA, Hackett KT, Dillard JP. The lytic transglycosylases of Neisseria gonorrhoeae. Microb Drug Resist 2012;18:271-9. [Crossref] [PubMed]
- Schaub RE, Chan YA, Lee M, et al. Lytic transglycosylases LtgA and LtgD perform distinct roles in remodeling, recycling, and releasing peptidoglycan in Neisseria gonorrhoeae. Mol Microbiol 2016;102:865-81. [Crossref] [PubMed]
- Campbell R. The psychological impact of rape victims. Am Psychol 2008;63:702-17. [Crossref] [PubMed]
- Cromwell PF, Risser WL, Risser JM. Prevalence and incidence of pelvic inflammatory disease in incarcerated adolescents. Sex Transm Dis 2002;29:391-6. [Crossref] [PubMed]
- Patel A, DeLong G, Voigl B, et al. Pelvic inflammatory disease in the lesbian population—lesbian health issues: asking the right questions. Obstet Gynecol 2000;95:S29-30. [Crossref]
- Coker TR, Austin SB, Schuster MA. The health and health care of lesbian, gay, and bisexual adolescents. Annu Rev Public Health 2010;31:457-77. [Crossref] [PubMed]
- Chelvakumar G, Gannon-Loew K. Sexually transmitted infections in lesbian, gay, bisexual, transgender, and questioning youth. Adolesc Med State Art Rev 2018;29:44-64.
- Forsyth S, Rogstad K. Sexual health issues in adolescents and young adults. Clin Med (Lond) 2015;15:447-51. [Crossref] [PubMed]
- Derniaux E, Lucereau-Barbier M, Graesslin O. Follow-up and counselling after pelvic inflammatory disease. J Gynecol Obstet Biol Reprod (Paris) 2012;41:922-9. [Crossref] [PubMed]
- Workowski KA, Bolan GACenters for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64:1-137. [PubMed]
- Wiesenfeld HC, Hillier SL, Krohn MA, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol 2002;100:456-63. [PubMed]
- Wiesenfeld HC, Hllier SL, Meyn LA, et al. Subclinical pelvic inflammatory disease and infertility. Obstet Gynecol 2012;120:37-43. [Crossref] [PubMed]
- Simon EM, April MD. Fitz-Hugh-Curtis syndrome. J Emerg Med 2016;50:e197-8. [Crossref] [PubMed]
- Muschart X. A case report with Fitz-Hugh-Curtis syndrome, what does it mean? Acta Clin Belg 2015;70:357-8. [Crossref] [PubMed]
- Chung HJ, Choi HY, Cho YJ, et al. Ten cases of Fitz-Hugh-Curtis syndrome. Korean J Gastroenterol 2007;50:328-33. [PubMed]
- Antonie F, Billiou C, Vic P. Chlamydia trachomatis Fitz-Hugh-Curtis syndrome in a female adolescent. Arch Pediatr 2013;20:289-91. [Crossref] [PubMed]
- Mitaka H, Kitazono H, Deshpande GA, et al. Fitz-Hugh-Curtis syndrome lacking typical characteristics of pelvic inflammatory disease. BMJ Case Rep 2016; [Crossref] [PubMed]
- Gaitán H, Angel E, Diaz R, et al. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol 2002;10:171-80. [Crossref] [PubMed]
- Haggerty CL, Ness RB. Newest approaches to treatment of pelvic inflammatory disease: a review of recent randomized clinical trials. Clin Infect Dis 2007;44:953-60. [Crossref] [PubMed]
- Erenel H, Yilmaz N, Oncul M, et al. Usefulness of serum procalcitonin levels in predicting tubo-ovarian abscess in patients with acute pelvic inflammatory disease. Gynecol Obstet Invest 2017;82:262-6. [Crossref] [PubMed]
- Kielly M, Jamieson MA. Pelvic inflammatory disease in virginal adolescent females without tubo-ovarian abscess. J Pediatr Adolesc Gynecol 2014;27:e5-7. [Crossref] [PubMed]
- Goodwin K, Fleming N, Dumont T. Tubo-ovarian abscess in virginal adolescent females: a case report and review of the literature. J Pediatr Adolesc Gynecol 2013;26:e99-102. [Crossref] [PubMed]
- Simpson-Camp L, Richardson EJ, Alaish SM. Streptococcus viridans tubo-ovarian abscess in an adolescent virgin. Pediatr Int 2012;54:706-9. [Crossref] [PubMed]
- Greydanus DE, Seyler J, Omar HA. Sexually transmitted diseases (STDs). In: Adolescent Medicine: Pharmacotherapeutics in General, Mental, and Sexual Health. Berlin/Boston: De Gruyter, 2012:338.
- Cho HW, Koo YJ, Min KJ, et al. Pelvic Inflammatory Disease in Virgin Women With Tubo-ovarian Abscess: A Single-Center Experience and Literature Review. J Pediatr Adolesc Gynecol 2017;30:203-8. [Crossref] [PubMed]
- WHO Model Prescribing Information: Drugs used in Bacterial Infections, 2001. Available online: https://apps.who.int/medicinedocs/en/d/Js5406e/
- WHO guidelines for the treatment of Neisseria gonorrhoeae. Geneva: World Health Organization, WHO Guidelines Approved by the Guidelines Review Committee, WHO Press, 1211 Geneva 27, Switzerland, 2016.
- Centers for Disease Control and Prevention (CDC). Update to CDC's sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56:332-6. [PubMed]
- Munoz JL, Goje OJ. Mycoplasma genitalium: an emerging sexually transmitted infection. Scientifica (Cairo) 2016;2016:7537318. [Crossref] [PubMed]
- Jensen JS, Cusini M, Gomberg M, et al. Background review for the 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol 2016;30:1686-93. [Crossref] [PubMed]
- Leli C, Mencacci A, Bombaci JC, et al. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in a population of Italian and immigrant outpatients. Infez Med 2012;20:82-7. [PubMed]
- Wiske CP, Palisoul M, Tapé C, et al. Physician specialty influences care of pelvic inflammatory disease. J Womens Health (Larchmt) 2016;25:723-8. [Crossref] [PubMed]
- Goyal M, Hersh A, Luan X, et al. Are emergency departments appropriately treating adolescent pelvic inflammatory disease? JAMA Pediatr 2013;167:672-3. [Crossref] [PubMed]
- Shih TY, Gavdos CA, Rothman RE, et al. Poor provider adherence to the Centers for Disease Control and Prevention treatment guidelines in US emergency visits with a diagnosis of pelvic inflammatory disease. Sex Transm Dis 2011;38:299-305. [PubMed]
- Llata E, Bernstein KT, Kerani RP, et al. Management of Pelvic Inflammatory Disease in Selected U.S. Sexually Transmitted Disease Clinics: Sexually Transmitted Disease Surveillance Network, January 2010-December 2011. Sex Transm Dis 2015;42:429-33. [Crossref] [PubMed]
- Weström L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility. A cohort of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992;19:185-92. [Crossref] [PubMed]
-
World Health Organization Medical Eligibility Criteria for Contraceptive Use 2015 . - Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep 2016;65:1-103. [PubMed]
- Horrow MM. Ultrasound of pelvic inflammatory disease. Ultrasound Q 2004;20:171-9. [Crossref] [PubMed]
- US Preventive Services Task Force. Screening for chlamydial infection: recommendations and rationale. Am J Prev Med 2001;20:90-4. [PubMed]
- Meyer T. Diagnostic Procedures to Detect Chlamydia trachomatis Infections. Microorganisms 2016; [Crossref] [PubMed]
- Abu Raya B, Bamberger E, Kerem NC, et al. Beyond “safe sex”—can we fight adolescent pelvic inflammatory disease? Eur J Pediatr 2013;172:581-90. [Crossref] [PubMed]
- Scholes D, Stergachis A, Heidrich FE, et al. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med 1996;334:1362-6. [Crossref] [PubMed]
- Bender N, Hermann B, Andersen B, et al. Chlamydia infection, pelvic inflammatory disease, ectopic pregnancy and infertility: cross-national study. Sex Transm Infect 2011;87:601-8. [Crossref] [PubMed]
- Aghaizu A, Adams EJ, Turner K, et al. What is the cost of pelvic inflammatory disease and how much could be prevented by screening for Chlamydia trachomatis? Cost analysis of the Prevention of Pelvic Infection (POPI) trial. Sex Transm Infect 2011;87:312-7. [Crossref] [PubMed]
- Gottlieb SL, Xu F, Brunham RC. Screening and treating Chlamydia trachomatis genital infection to prevent pelvic inflammatory disease: interpretation of findings from randomized control trials. Sex Transm Dis 2013;40:97-102. [Crossref] [PubMed]
- Greydanus DE, Pratt HD. Human sexuality. Internat J Child Adolesc Health 2016;9:11-7.
Cite this article as: Greydanus DE, Bacopoulou F. Acute pelvic inflammatory disease: a narrative review. Pediatr Med 2019;2:36.

