
Long-acting reversible contraception for adolescents
Introduction
Adolescents continue to be sexually active, with forty two percent of 15–19 year old females in the United States reported to have ever engaged in vaginal intercourse with opposite-sex partner (1). Despite 99.4% of sexually active females reporting ever-using contraception, 75% of pregnancies in this age group during 2011 were unintended (1,2). Condoms, withdrawal, and oral contraceptive pills were the most commonly reported methods of contraception used by female teens during this period (97.4%, 59.7%, and 55.5% respectively) (1). Different user dependent contraceptive methods result in decreased effectiveness due to user errors with typical use. A 2017 study, analyzing data extracted from the National Survey of Family Growth in the United States from 2006-2010 showed that withdrawal, condom, and oral contraceptive pills had a significantly higher probability of failure within 1 year of use compared to non-user dependent long-acting reversible contraception (LARC) (3). Due to their safety and effectiveness, LARC is recommended for use in adolescents by the American College of Obstetricians and Gynecologists, the Center for Disease Control and Prevention, the Society for Adolescent Health and Medicine, and the Society of Family Planning (4-9). LARC is considered a first-line contraceptive choice for adolescents and young adults by the American Academy of Pediatrics; however, LARC was used only by 5.8% of 15–19-year-old females between 2011 and 2015, with 2.8% using intrauterine devices (IUDs) and 3% using the implant (1,10).
Long-acting reversible contraceptive methods
The copper T380A intrauterine device
The copper T380A IUD (ParaGard; CooperSurgical Inc., Trumbull, CT, USA) is a T-shaped device, approximately 32 mm x 36 mm, that is inserted into the uterus through the cervix (7,11). It is made of polyethylene and wrapped with 313.4 mg of copper wire (7,11). The frame contains barium sulfate and is radiopaque (7,11). A monofilament tread extends from the 3 mm diameter tip to aid in detection and removal of the device (11). It is approved by the United States Food and Drug Administration (FDA) for up to 10 years of contraceptive use (7,12).
The hormonal subdermal implant
The etonogestrel subdermal implant (Nexplanon: Merck Sharpe and Dohme, Whitehouse Station, New Jersey, USA) is a 40 mm rod with a 2 mm diameter made of a flexible ethylene vinyl acetate core containing 68 mg of etonogestrel and surrounded by a membrane responsible for the controlled release of etonogestrel (ENG) over 3 years (12,13). It is inserted beneath the dermis on the medial aspect of the upper arm and contains barium sulfate, making it radiopaque (13). The 68 mg of ENG is released gradually starting at 60–70 mcg/day, progressively decreasing to 25–30 µg/day by the third year of use (14).
Hormonal intrauterine devices
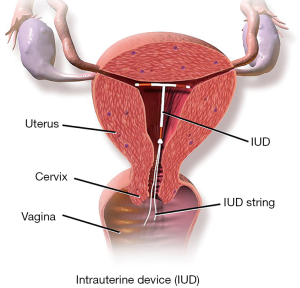
The levonorgestrel intrauterine device (LNG-IUD) consists of a T-shaped device, approximately 32 mm × 32 mm, that is inserted into the uterus through the cervix (7,15,16). It is made of polyethylene and contains a hormone reservoir around the vertical portion of the device (7,15,16). The reservoir is made of levonorgestrel and silicone, and contains a specific amount of levonorgestrel to be released gradually overtime (7,15,16). The reservoir is surrounded by a membrane that controls the gradual release of hormone (7,15,16). The frame contains barium sulfate, making it radiopaque (7,15,16). A monofilament thread extends from the vertical stem to aid in detection and removal of the device (15). There are four types of LNG-IUDs available in the United States market, each containing a different amount of levonorgestrel and different rate of LNG release (Table 1) (17-22). Figure 1 depicts a typical IUD in place.
Table 1
| LNG-IUD (total LNG dose) | US brand name1 | Effective duration | Initial rate of LNG release | Average rate of LNG release |
|---|---|---|---|---|
| LNG-IUD (19.5 mg) | Kyleena | 5 years | 17.5 μg/day | 9 μg/day over 5 years |
| LNG-IUD (52 mg) | Liletta | 5 years | 19.5 μg /day | 14.7 μg/day over 5 years |
| LNG-IUD (52 mg) | Mirena | 5 years | 20 μg/day | 20 μg/day over 5 years |
| LNG-IUD (13.5 mg) | Skyla | 3 years | 14 μg/day | 8 μg/day over 3 years |
1, US brands: Kyleena, Mirena, and Skyla, manufactured by Bayer HealthCare Pharmaceuticals, Ind, Whippany, USA. Liletta, manufactured by Allergan, Irvine, California, USA.
Mechanisms of action of LARC methods
LARC methods provide effective contraception by preventing fertilization by different underlying mechanisms of action (Table 2) (23-35). With the ENG subdermal implant, the primary mechanism of action is preventing ovulation. ENG interferes with the hypothalamic-pituitary axis by preventing the luteinizing hormone (LH) surge, resulting in anovulation (23). Anovulation is responsible for 99% of the contraceptive action of the ENG subdermal implant (23). The remaining 1% is due to decreased sperm motility secondary to increased viscosity of cervical mucus, and by thinning the endometrium (23-25).
Table 2
| Method | Primary mechanism | Secondary mechanism |
|---|---|---|
| ENG subdermal implant | Prevention of ovulation by diminishing LH surge | Decreased sperm motility by increased cervical mucus viscosity and thinning of endometrium |
| LNG-IUD | Thickening of cervical mucus, impaired ability of sperm to fertilize ovum, decidualization and atrophy of endometrial glands | Sterile inflammatory reaction of the endometrium to the presence of IUD, phagocytosis of sperm in the endometrial cavity |
| Copper IUD | Spermicidal effect of copper, inhibition of sperm mobility, inhibition of ovum migration, direct toxic effect of copper on sperm | Sterile inflammatory reaction of the endometrium to the presence of IUD, phagocytosis of sperm in the endometrial cavity |
LARC, long-acting reversible contraceptive; ENG, etonogestrel; LH, luteinizing hormone; LNG-IUD, levonorgestrel intrauterine device.
An IUD present in the uterus is recognized as a foreign body by the immune system and in response, the number of leukocytes in the endometrial cavity and fallopian tubes increases (26,27). The leukocytes can then phagocytose sperm (26,27). Studies have shown that sperms in the endometrial cavity are phagocytosed within 16 hours after intercourse due to this sterile inflammatory response (28). This likely explains the low number of sperms found in fallopian tubes after intercourse in women using IUDs (26).
With the copper IUD, the primary mechanisms of action are its spermicidal effect, inhibition of sperm motility, and inhibition of ovum migration (29,30). A higher copper level in the IUD is associated with increased contraceptive efficacy (29). Copper is released from the IUD into the uterine cavity. When sperm is present, the copper binds tightly to it, decreasing motility and viability (31). Copper binding to sperm can result in the head of the sperm separating from its tail (31,32). Copper causes an enhanced inflammatory response, stronger than the foreign body inflammatory response seen with inert and hormonal IUDs (27). This response leads to further destruction of sperms (29). Copper ions in the intrauterine space alter the endometrium, further decreasing sperm viability and motility (30). A high copper concentration is also found in the cervical mucus (30,33,34) and can inhibit sperm motility (30,35). Studies suggest copper may be similarly toxic to the ovum (32,36). This could explain the finding of fewer ova in the fallopian tubes of copper IUD users, likely caused by leukocytes destroying the ovum as it travels through the fallopian tube (32,36). It is unlikely that the copper IUDs impairs implantation of the blastocysts after fertilization, and there is no evidence to support that the copper IUD is an abortifacient (27,30,33,37,38).
The active ingredient in the LNG-IUD is the synthetic steroid levonorgestrel, a second-generation progestin (39). LNG binds to the progesterone receptor with 3.2 times the relative affinity of in vivo progesterone (40,41). Studies show that when LNG is released in the uterus via the IUD, there is a high specific endometrial uptake, while serum concentration remains low (42,43). With the LNG-IUD, the primary mechanism of action is to thicken the cervical mucus, impairing the ability of sperm to fertilize the ovum (44-47). Additionally, LNG causes apoptosis of the endometrial layer by causing an increased expression of Fas antigen and decreased expression of Bcl-2 protein in the cells of the endometrium (27,48). This mechanism causes a decrease in endometrial proliferation and is likely responsible for reducing menses and the effectiveness of the LNG-IUD in treating menorrhagia (27,48). It is unclear if this disruption to the endometrium has the ability to inhibit blastocyst implantation (16). Similar to the copper IUD, there is no evidence that the LNG-IUD is an abortifacient (27,33).
High serum levels of LNG are required to suppress ovulation (16). Given that most of the LNG released by the IUD is taken up by the endometrium, with a low amount entering the serum, an intrauterine dose of 50 µg/day of LNG would be required to raise serum LNG sufficient to cause anovulation (16). The highest concentration of LNG released from currently used IUDs is not more than 20 µg/day. If anovulation is to occur, it is most likely to occur shortly after the IUD is placed, as the concentration of LNG released per day decreases over time. Given the same daily dose of LNG, four different cycle reactions have been described: (I) anovulation, (II) anovulation with high follicular activity, (III) ovulation with luteal insufficiency, and (IV) normal ovulation (45,46). Early after LNG-IUD insertion, when systemic LNG is higher, cycles can be anovulatory in some women (45,46). Most cycles become ovulatory after the first year of use (45,46,49).
LNG causes a local decrease in prostaglandin production, causing decreased motility of the fallopian tubes and a shortened fertility window (16). The foreign body response triggered by the IUD induces an inflammatory response that results in ovum and sperm apoptosis (16). These mechanisms play an important, but minor role in contraception.
Of the currently available hormonal IUDs, the LNG-IUD 13.5 mg contains the lowest amount of LNG. Serum LNG levels, while on LNG-IUD 13.5 mg are not high enough to suppress ovulation in most women (19). Although, systemic LNG and rates of anovulation were lower with the LNG-IUD 13.5 mg compared to the LNG-IUD 52 mg, the effect of LNG on endometrial proliferation and cervical mucus production remains the same (19). While the degree of ovulatory suppression varies between LNG-IUDs, the degree of cervical mucus thickening is comparable between the LNG-IUD 13.5 mg, 19.5 mg, and 52 mg (16). Thickening of cervical mucus creates a barrier to sperm penetration (29,50). Decidualization and atrophy of the endometrial glands can reduce sperm survival (29,51).
Effectiveness of LARC methods
LARC is the most effective form of birth control for women. Contraceptive effectiveness refers to how well a pregnancy is prevented with the typical use of a method, while efficacy refers to how well a pregnancy is prevented with the perfect use of a method (52). Many of the non-barrier contraceptive methods have similar efficacy; but, because of the varying degree of user involvement with each method, the effectiveness differs significantly between non-barrier contraceptive options (52). LARC and sterilization require no user involvement; and, proper and consistent use is essentially guaranteed. These methods have nearly identical efficacy and effectiveness. However, methods such as the pill, the injectable, and the condom require different degrees of user involvement, and allow for potential imperfect use. This potential user error results in reduced effectiveness compared to reported efficacy.
The Pearl Index is used to compare the effectiveness of different birth control methods and represents the number of contraceptive failures per 100 women-years of use (52). The most effective form of birth control is the hormonal subdermal implant. The Pearl Index of the ENG implant reported from the Trussell study is 0.05 (52). However, subsequent studies reported that the implant may have a higher effectiveness. A study of 24,100 cycles in 923 women suggests that the Pearl Index of the subdermal implant is 0.006 (53). Pearl Indices for LARC methods available in the United States are listed in Table 3 (20,52,54,55). A study of 61,488 women from 2006-2012 suggests that the Pearl Indices of LNG-IUDs and copper IUDs may be lower than typically quoted with a Pearl Index of collective LNG-IUDs of 0.06 (95% CI: 0.04–0.09) and a Pearl Index of copper IUDs of 0.52 (95% CI: 0.42–0.64) (56).
Table 3
| LARC method | Pearl index, per 100 women-years |
|---|---|
| ENG subdermal implant (Implanon) | 0.05 (52) |
| LNG-52 mg (Mirena) | 0.20 (52) |
| LNG-19.5 mg (Kyleena) | 0.31 (95% CI: 0.15–0.57) (20) |
| LNG-52 mg (Liletta) | 0.22 (95% CI 0.08–0.49) (54) |
| LNG-13.5 mg (Skyla) | 0.33 (95% CI: 0.16–0.60) (20,55) |
| Copper T380A (ParaGard) | 0.80 (52) |
1, US brands: Kyleena, Mirena, and Skyla, manufactured by Bayer HealthCare Pharmaceuticals, Ind, Whippany, USA. Liletta, manufactured by Allergan, Irvine, California, USA. Paragard manufactured by CooperSurgical Inc., Trumbull, CT, USA; Implanon manufactured by Merck Sharpe and Dohme, Whitehouse Station, New Jersey, USA. LARC, long-acting reversible contraceptive; LNG, levonorgestrel; ENG, etonogestrel.
When the Pearl Indices are compared, there is no significant difference in pregnancy rates over time between different LNG-IUDs (20). It has also been shown that different LNG-IUDs are similarly effective regardless of age, parity, and BMI (55). Studies have also shown that the LNG subdermal implant is effective in overweight and obese women (57). Based on data collected from the National Survey of Family Growth from 2006-2010, LARC had the lowest failure rates of all contraceptive methods, with a combined failure rate of 1% (3).
Safety of LARC methods
Overall, studies continue to show that the ENG subdermal implant, the LNG-IUD, and the copper IUD are safe for use in adolescent and young adult women, including nulliparous women. The US Medical Eligibility Criteria for Contraceptive Use categorizes ENG subdermal implant as safe for nulliparous women to use without restrictions (8,58). It categorizes use of both the LNG-IUD and the copper IUD in nulliparous women as having advantages that outweigh theoretical or proven risks (8,58). Contraindications to the use of each method are listed in Table 4 (8,58,59). Major safety concerns regarding LARC pertain almost exclusively to IUDs and include: device expulsion, uterine perforation, pelvic inflammatory disease (PID), ectopic pregnancy, and infertility after discontinuation of the method.
Table 4
| ENG subdermal implant |
| Current pregnancy |
| Acute liver disease |
| Undiagnosed abnormal uterine bleeding |
| Breast cancer (current diagnosis or history) |
| Hypersensitivity reaction to components of the implant |
| LNG-IUD |
| Current pregnancy |
| PID within the last 3 months |
| Acute cervicitis |
| Post-partum or post-abortion sepsis within the last 3 months |
| Undiagnosed abnormal uterine bleeding |
| Genital tract malignancy |
| Uterine anomaly |
| Breast cancer (current diagnosis or history) |
| Copper IUD |
| Current pregnancy |
| PID within the last 3 months |
| Acute cervicitis |
| Post-partum or post-abortion sepsis within the last 3 months |
| Undiagnosed abnormal uterine bleeding |
| Genital tract malignancy |
| Uterine anomaly |
| Wilson’s disease |
LARC, long-acting reversible contraceptive; LNG-IUD, levonorgestrel intrauterine device; ENG, etonogestrel; PID, pelvic inflammatory disease.
IUD expulsion is rare and occurs at similar rates regardless of IUD type (Table 5) (20,54,55,60-65). IUD expulsion is not technically a safety issue, but it does increase the risk of unintended pregnancy (60). One study found that the odds ratio of increased IUD failure with IUD expulsion was 3.31 (95% CI: 1.40–7.81) (61). While the rates of uterine expulsion between nulliparous and parous women using the LNG-IUD were similar, rates of uterine expulsion were slightly higher for nulliparous women compared to parous women using the copper IUD (62). There is an increased risk of expulsion of the copper IUD if it is reinserted after an expulsion (63). The rate of expulsion increases if the first expulsion occurs within the first three months after IUD placement (41% vs. 18%, P=0.001) (63). Risk of copper IUD expulsion decreases with age (60). Expulsion was more likely to occur in parous compared to nulliparous women with LNG 19.5 and LNG-52 (55,64). Expulsion of LNG-52 was not affected by parity (65).
Table 5
| IUD1 | Rate of uterine expulsion, % |
|---|---|
| LNG-52 mg (Mirena) | 1.6 (65) |
| LNG-19.5 mg (Kyleena) | 3.6 (20,55) |
| LNG-52 mg (Liletta) | 3.5 (54) |
| LNG-13.5 mg (Skyla) | 4.6 (20,55) |
| Copper T380A (ParaGard) | 4.9 (65) |
1, US brands: Kyleena, Mirena, and Skyla, manufactured by Bayer HealthCare Pharmaceuticals, Ind, Whippany, USA. Liletta, manufactured by Allergan, Irvine, California, USA. Paragard manufactured by CooperSurgical Inc., Trumbull, CT, USA. IUD, intrauterine device; LNG, levonorgestrel.
Uterine perforation by LNG-IUDs and copper IUDs is rare. Multiple studies show uterine perforation affects between 0-1.3percent of women using IUDs (60,65-67). Perforation is most likely to occur during insertion of the IUD. A study of 61,448 women from 2006 to 2013 found uterine perforation occurred with 1.4 per 1000 insertions of the LNG-IUD (95% CI: 1.1–1.8) (68). It found that uterine perforation occurred in 1.1 per 1,000 insertions of the copper IUD. (95% CI: 0.7–1.7) (68).
Pelvic inflammatory disease (PID) as a result of an IUD use is rare, with rates ranging from 0–2.5% (65,69-71). This rate is comparable to the rate of PID found with the use of ENG implant, oral contraceptive pill, and depo-medroxyprogesterone injection (60,72). There is an increased risk of PID during the first 20 days after insertion of an IUD if a chlamydia or gonorrhea infection is present at the time of insertion (73). This is thought to be due to the entrance of existing vaginal bacteria into the uterus via direct contact with the IUD during insertion (73). PID risk is not increased in nulliparous women (62). A randomized trial of 2,500 women found that LNG-IUD significantly lowered the risk of PID compared to the copper IUD and non-IUD users (62,74). It also found PID rates to be comparable in copper IUD and non-IUD users (62,74). The protective effects of the LNG-IUD could be explained by the endometrial suppression and cervical mucus thickening that is responsible for the method’s contraceptive effects (74).
While the relative risk of ectopic pregnancy increases with IUD use, the absolute risk decreases due to the effectiveness of IUDs at preventing pregnancy (65-66,70,75,76). A study of 61,448 women from 2006 to 2012 calculated the risk of ectopic pregnancy to be 0.06 per 100 women-years (95% CI: 0.04–0.09) for the LNG-IUD, and 0.52 per 100 women-years (95% CI: 0.42–0.64) for the cooper IUD (56). Absolute ectopic pregnancy risk is lower with the LNG-IUD compared to the copper IUD. The hazard ratio for ectopic pregnancies with LNG-IUD and copper IUD use is 0.26 (95% CI: 0.10–0.66) (56).
Fertility returns rapidly after implant and IUD removal (62,70,77-80). Studies have found no overall difference in 12-month pregnancy rates with IUD users compared to non-IUD users after removal of the IUD (81). The copper IUD is not a risk factor for tubal occlusion leading to infertility (82).
Studies suggest that the ENG implant, LNG-IUD, and copper IUD do not negatively affect bone mineral density and can be safely used in adolescents who have not reached peak bone mass (83-85). LNG-IUD use may be associated with reduced fracture risk (86).
A change in menstrual pattern and irregular menstrual periods are a common concern, especially during the first year of ENG subdermal implant use (86,87). Other side effects associated with the use of ENG subdermal implant include headache, weight gain, acne, dizziness, depressed mood, nausea, lower abdominal pain, hair loss, loss of libido, and an increased risk for the development of ovarian follicular cysts (86,87). Rare safety concerns are associated with the procedure of insertion and removal of the implant. In general, subdermal implant is well accepted and most side effects are infrequent and rarely require discontinuation or removal of the device (86,87).
Use of LARC methods by adolescents
Data from 2015 suggests that only 3.4% of adolescents in the United States use a LARC method (95% CI: 2.9–3.9) (88). This study found that older adolescents are more likely to use LARC than younger adolescents, with an adjusted odds ratio of 2.41 (95% CI: 1.62–3.58) comparing 20–21 and 15–17 years old (88). A number of factors have been identified as barriers (Table 6) or facilitators (Table 7) for the adoption and use of a LARC method by adolescents (64,71,88-99).
Table 6
| Domain | Barriers |
|---|---|
| Medical practitioner factors | Perception that LARC not appropriate for adolescents |
| LARC not offered due to safety concerns | |
| Belief that IUDs should not be used in nulliparous females | |
| Previous STI and multiple sex partners incorrectly considered as contraindication | |
| Perception that adolescents not interested in LARC | |
| Lack of training for LARC placement | |
| Lack of confidence in ability to adequately counsel regarding LARC | |
| Belief that LARC methods are traumatic to adolescents | |
| Do not feel that they can keep up with clinical skills needed for LARC use | |
| Adolescent factors | Not offered LARC option or counselled by their physician |
| Fear of complications with IUD placement and use | |
| Fear of pain with insertion or removal of IUD or implant | |
| Fear of limitation of physical activity with IUD use | |
| Fear of expulsion of IUD, future infertility | |
| Fear of future fertility | |
| Concern about weight gain, and irregular bleeding | |
| Lack of anatomical knowledge to understand LARC and associated risks | |
| Misconception that LARC is not effective | |
| Concern about consent and confidentiality | |
| Belief that parental permission was needed for LARC placement | |
| Cost | |
| Concern that IUDs should not be used in nulliparous females | |
| System factors | Physician office not set up for LARC method placement |
| Expense may not be covered by health system | |
| Insufficient access to physicians who will insert IUD or subdermal implant | |
| LARC method may not be readily available or in stock in the clinic or office | |
| Practice of requiring separate appointments for contraceptive counseling and LARC placement |
LARC, long-acting reversible contraceptive; IUD, intrauterine device; STI, sexually transmitted infection.
Table 7
| Longer duration of method’s effectiveness |
| Lack of user dependency with LARC methods |
| Increased knowledge regarding eligibility for LARC awareness efforts |
| Assurance of confidentiality |
| Elimination of cost barriers |
| Dispelling of misconceptions about risks and side effects |
| Acceptability and use by adolescent’s social circle |
| Adolescent’s personal acceptability of the method |
| History of prior pregnancy |
| Improved access |
LARC, long-acting reversible contraceptive.
Conclusions
LARC methods are the recommended methods of choice for contraception in adolescents and young adult women. The ENG subdermal implant, the LNG-IUD, and the copper IUD are safe and effective methods of contraception for adolescents and young adult women. The US Medical Eligibility Criteria for Contraceptive Use categorizes implant as safe for nulliparous women to use without restrictions. It categorizes use of both the LNG-IUD and the copper IUD in nulliparous women as having advantages that outweigh theoretical or proven risks. With appropriate education and training, medical practitioners in their primary care medical practice settings can effectively use LARC methods.
There needs to be better education and training for medical practitioners regarding the use of LARC methods including training in the proper procedures for insertion and removal of subdermal implant and IUDs. Training on LARC placement should be easily accessible to all medical practitioners who wish to make this a part of their practice. Additionally, adolescents and young adult women need education about LARC. Contraceptive counseling should include LARC methods, even if the adolescent did not request them. Medical practitioners should provide factual, non-biased information regarding all contraceptive methods, and use factors that the adolescent has identified as valuable in a birth control method to guide the discussion. Barriers associated with cost and confidentiality should be addressed at the policy level.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Maria Demma Cabral and Dilip R. Patel) for the series “Adolescent Gynecology” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.07.10). The series “Adolescent Gynecology” was commissioned by the editorial office without any funding or sponsorship. DRP served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Pediatric Medicine from Jul 2018 to Jun 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abma JC, Martinez GM. Sexual Activity and Contraceptive Use Among Teenagers in the United States, 2011-2015. Natl Health Stat Report 2017;1-23. [PubMed]
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med 2016;374:843-52. [Crossref] [PubMed]
- Sundaram A, Vaughan B, Kost K, et al. Contraceptive failure in the United States: estimates from the 2006-2010 National Survey of Family Growth. Perspect Sex Reprod Health 2017;49:7-16. [Crossref] [PubMed]
- Committee on Adolescent Health Care. ACOG Committee Opinion No. 735: Adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol 2018;131:e130-9. [Crossref] [PubMed]
- Committee on Adolescent Health Care. Committee Opinion No 699: Adolescent Pregnancy, Contraception, and Sexual Activity. Obstet Gynecol 2017;129:e142-9. [Crossref] [PubMed]
- Committee on Adolescent Health Care. Committee Opinion No. 710: Counseling Adolescents About Contraception. Obstet Gynecol 2017;130:e74-80. [Crossref] [PubMed]
- Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice Bulletin No. 186: Long-Acting Reversible Contraception: Implants and Intrauterine Devices. Obstet Gynecol 2017;130:e251-69. [PubMed]
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016;65:1-103. [PubMed]
- Society for Adolescent Health and Medicine. Improving Knowledge About, Access to, and Utilization of Long-Acting Reversible Contraception Among Adolescents and Young Adults. J Adolesc Health 2017;60:472-4. [Crossref] [PubMed]
- Ott MA, Sucato GS. Contraception for adolescents. Committee on Adolescence. Pediatrics 2014;134:e1244-56. [Crossref]
- ParaGard® T 380A Intrauterine Copper Contraceptive. Available online: https://www.paragard.com/pdf/PARAGARD-PI.pdf. accessed June 9, 2019
- Francis JKR, Gold MA. Long-Acting Reversible Contraception for Adolescents: A Review. JAMA Pediatr 2017;171:694-701. [Crossref] [PubMed]
- Available online: https://www.merck.com/product/usa/pi_circulars/n/nexplanon/nexplanon_pi.pdf. accessed June 9, 2019.
- Espey E, Ogburn T. Long-acting reversible contraceptives: intrauterine devices and the contraceptive implant. Obstet Gynecol 2011;117:705-19. [Crossref] [PubMed]
- Mirena® (levonorgestrel-releasing intrauterine system). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021225s019lbl.pdf. accessed June 9, 2019.
- Grandi G, Farulla A, Sileo FG, et al. Levonorgestrel-releasing intrauterine systems as female contraceptives. Expert Opin Pharmacother 2018;19:677-86. [Crossref] [PubMed]
- Rowe P, Farley T, Peregoudov A, et al. IUD Research Group of the UNDP/UNFPA/WHO/World Bank Special Programme of Research: Development and Research Training in Human Reproduction. Safety and efficacy in parous women of a 52 mg levonorgestrel-medicated intrauterine device: a 7 year randomized comparative study with the TCu380A. Contraception 2016;93:498-506. [Crossref] [PubMed]
- Skyla (levonorgestrel-releasing intrauterine system). Highlights of prescribing information. Whippany (NJ): Bayer HealthCare Pharmaceuticals Inc. 2017. Available online: http://labeling.bayerhealthcare.com/html/products/pi/ Skyla_PI.pdf. Retrieved August 23, 2017.
- Apter D, Gemzell-Danielsson K, Hauck B, et al. Pharmacokinetics of two low-dose levonorgestrel-releasing intrauterine systems and effects on ovulation rate and cervical function: pooled analyses of phase II and III studies. Fertil Steril 2014;101:1656-62.e1-4.
- Nelson A, Apter D, Hauck B, et al. Two low-dose levonorgestrel intrauterine contraceptive systems: a randomized controlled trial. Obstet Gynecol 2013;122:1205-13. [Crossref] [PubMed]
- Creinin MD, Jansen R, Starr RM, et al. Levonorgestrel release rates over 5 years with the Liletta 52 mg intrauterine system. Contraception 2016;94:353-6. [Crossref] [PubMed]
- Kyleena (levonorgestrel-releasing intrauterine system). Highlights of prescribing information. Whippany (NJ): Bayer HealthCare Pharmaceuticals Inc.; 2016. Available online: http://labeling.bayerhealthcare.com/html/ products/pi/Kyleena_PI.pdf. Retrieved August 23, 2017. (Level III).
- Croxatto HB. Mechanisms that explain the contraceptive actions of progrestin implants for women. Contraception 2002;65:21-7. [Crossref] [PubMed]
- Davies GC, Feng LX, Newton JR, et al. Release characteristics, ovarian activity and menstrual bleeding pattern with a single contraceptive implant releasing 3-ketodesogestrel. Contraception 1993;47:251-61. [Crossref] [PubMed]
- Van den Bosch T, Donders GG, Riphagen I, et al. Ultrasonographic features of the endometrium and the ovaries in women on etonogestrel implant. Ultrasound Obstet Gynecol 2002;20:377-80. [Crossref] [PubMed]
- El-Habashi M, El-Sahwi S, Gawish S, et al. Effect of Lippes loop on sperm recovery from human fallopian tubes. Contraception 1980;22:549-55. [Crossref] [PubMed]
- Ortiz ME, Croxatto HB. Copper-T intrauterine device and levonorgestrel intrauterine system: biological bases of their mechanism of action. Contraception 2007;75:S16-30. [Crossref] [PubMed]
- Sağiroğlu N. Phagocytosis of spermatozoa in the uterine cavity of woman using the intrauterine device. Int J Fertil 1971;16:1-14. [PubMed]
- Mishell DR. Intrauterine devices: mechanisms of action, safety, and efficacy. Contraception 1998;58:45S-53S. [Crossref] [PubMed]
- Kaneshiro B, Aeby T. Long-term safety, efficacy, and patient acceptability of the intrauterine Copper T-380A contraceptive device. Int J Womens Health 2010;2:211-20. [Crossref] [PubMed]
- Holland MK, White IG. Heavy metals and human spermatozoa. III. The toxicity of copper ions for spermatozoa. Contraception 1988;38:685-95. [Crossref] [PubMed]
- Sivin I. IUDs are contraceptives, not abortifacients: a comment on research and belief. Stud Fam Plann. 1989;20:355-9. [Crossref] [PubMed]
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002;187:1699-708. [Crossref] [PubMed]
- Hagenfeldt K. Intrauterine contraception with the copper-T device: effect on trace elements in the endometrium, cervical mucus and plasma. Contraception 1972;6:37-54. [Crossref] [PubMed]
- Hefnawi F, Kandil O, Askalani A, et al. Mode of action of the copper IUD: effect on endometrial copper and cervical mucus sperm migration. Proceedings of the Third International Conference of Intrauterine Contraception, 1974 Dec 12-14; Cairo, Egypt. New York: Elsevier, 1974.
- Alvarez F, Brache V, Fernandez E, et al. New insights on the mode of action of intrauterine contraceptive devices in women. Fertil Steril 1988;49:768-73. [Crossref] [PubMed]
- Segal SJ, Alvarez-Sanchez F, Adejuwon CA, et al. Absence of chorionic gonadotropin in sera of women who use intrauterine devices. Fertil Steril 1985;44:214-8. [Crossref] [PubMed]
- The TCu380A, TCu220C, multiload 250 and Nova T IUDS at 3,5 and 7 years of use--results from three randomized multicentre trials. World Health Organization. Special Programme of Research, Development and Research Training in Human Reproduction: Task Force on the Safety and Efficacy of Fertility Regulating Methods. Contraception 1990;42:141-58. [Crossref] [PubMed]
- Hümpel M, Wendt H, Pommerenke G, et al. Investigations of pharmacokinetics of levonorgestrel to specific consideration of a possible first-pass effect in women. Contraception 1978;17:207-20. [Crossref] [PubMed]
- Sitruk-Ware R. New progestogens for contraceptive use. Hum Reprod Update 2006;12:169-78. [Crossref] [PubMed]
- Grandi G, Mueller MD, Papadia A, et al. Inflammation influences steroid hormone receptors targeted by progestins in endometrial stromal cells from women with endometriosis. J Reprod Immunol 2016;117:30-8. [Crossref] [PubMed]
- Nilsson CG, Haukkamaa M, Vierola H, et al. Tissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUD. Clin Endocrinol (Oxf) 1982;17:529-36. [Crossref] [PubMed]
- French R, Gullebaud J. Mirena® the levonorgestrel intrauterine system (20 mcg/day). J Drug Evaluation 2003;1:43-69.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgesrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception 2017;95:464-9. [Crossref] [PubMed]
- Xiao B, Zeng T, Wu S, et al. Effect of levonorgestrel-releasing intrauterine device on hormonal profile and menstrual pattern after long-term use. Contraception 1995;51:359-65. [Crossref] [PubMed]
- Xiao BL, Zhou LY, Zhang XL, et al. Pharmacokinetic and pharmacodynamics studies of levonorgestrel-releasing intrauterine device. Contraception 1990;41:353-62. [Crossref] [PubMed]
- Natavio MF, Taylor D, Lewis RA, et al. Temporal changes in cervical mucus after insertion of the levonorgestrel- releasing intrauterine system. Contraception 2013;87:426-31. [Crossref] [PubMed]
- Maruo T, Laoag-Fernandez JB, Pakarinen P, et al. Effects of the levonorgestrel-releasing intrauterine system on proliferation and apoptosis in the endometrium. Hum Reprod 2001;16:2103-8. [Crossref] [PubMed]
- Luukkainen T, Allonen H, Haukkamaa M, et al. Five years’ experience with levonorgestrel-releasing IUDs. Contraception 1986;33:139-48. [Crossref] [PubMed]
- Lewis RA, Taylor D, Natavio MF, et al. Effects of the levonorgestrel-releasing intrauterine system on cervical mucus quality and sperm penetrability. Contraception 2010;82:491-6. [Crossref] [PubMed]
- Silverberg SG, Haukkamaa M, Arko H, et al. Endometrial morphology during long-term use of levonorgestrel-releasing intrauterine devices. Int J Gynecol Pathol 1986;5:235-41. [Crossref] [PubMed]
- Trussell J. Understanding contraceptive failure. Best Pract Res Clin Obstet Gynaecol 2009;23:199-209. [Crossref] [PubMed]
- Graesslin O, Korver T. The contraceptive efficacy of Implanon: a review of clinical trials and marketing experience. Eur J Contracept Reprod Health Care 2008;13:4-12. [Crossref] [PubMed]
- Eisenberg DL, Schreiber CA, Turok DKACCESS IUS investigators, et al. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception 2015;92:10-6. [Crossref] [PubMed]
- Gemzell-Danielsson K, Apter D, Hauck B, et al. The effect of age, parity and body mass index on the efficacy, safety, placement and user satisfaction associated with two low-dose levonorgestrel intrauterine contraceptive systems: subgroup analyses of data from a phase III trial. PLoS One 2015;10:e0135309 [Crossref] [PubMed]
- Heinemann K, Reed S, Moehner S, et al. Comparative contraceptive effectiveness of levonorgestrel-releasing and copper intrauterine devices: the European Active Surveillance Study for Intrauterine Devices. Contraception 2015;91:280-3. [Crossref] [PubMed]
- Xu H, Wade JA, Peipert JF, et al. Contraceptive failure rates of etonogestrel subdermal implants in overweight and obese women. Obstet Gynecol 2012;120:21-6. [Crossref] [PubMed]
- McNicholas C, Peipert JF. Long-acting reversible contraception for adolescents. Curr Opin Obstet Gynecol 2012;24:293-8. [Crossref] [PubMed]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol 2011;118:184-96. [Crossref] [PubMed]
- Jatlaoui TC, Riley HE, Curtis KM. The safety of intrauterine devices among young women: a systematic review. Contraception 2017;95:17-39. [Crossref] [PubMed]
- Thonneau P, Almont T, de La Rochebrochard E, et al. Risk factors for IUD failure: results of a large multicentre case-control study. Hum Reprod 2006;21:2612-6. [Crossref] [PubMed]
- Lyus R, Lohr P, Prager S. Use of the Mirena LNG-IUS and Paragard CuT380A intrauterine devices in nulliparous women. Contraception 2010;81:367-71. [Crossref] [PubMed]
- Bahamondes L, Diaz J, Marchi NM, et al. Performance of copper intrauterine devices when inserted after an expulsion. Hum Reprod 1995;10:2917-8. [Crossref] [PubMed]
- Madden T, McNicholas C, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol 2014;124:718-26. [Crossref] [PubMed]
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol 2014;123:585-92. [Crossref] [PubMed]
- Gemzell-Danielsson K, Buhling KJ, Dermout SM, et al. A Phase III, single-arm study of LNG-IUS 8, a low-dose levonorgestrel intrauterine contraceptive system (total content 13.5mg) in postmenarcheal adolescents. Contraception 2016;93:507-12. [Crossref] [PubMed]
- Sivin I, Stern J, Coutinho E, et al. Prolonged intrauterine contraception: a seven year randomized study of the levonorgestrel 20 mcg/day (LNG 20) and the Copper T380 Ag IUDs. (Evidence grade: I]). Contraception 1991;44:473-80. [Crossref] [PubMed]
- Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception 2015;91:274-9. [Crossref] [PubMed]
- Sivin I, Stern J. Health during prolonged use of levonorgestrel 20 micrograms/d and the copper TCu 380Ag intrauterine contraceptive devices: a multicenter study. International Committee for Contraception Research (ICCR). Fertil Steril 1994;61:70-7. [Crossref] [PubMed]
- Gemzell-Danielsson K, Apter D, Dermout S, et al. Evaluation of a new, low-dose levonorgestrel intrauterine contraceptive system over 5 years of use. Eur J Obstet Gynecol Reprod Biol 2017;210:22-8. [Crossref] [PubMed]
- Berenson AB, Tan A, Hirth JM, et al. Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol 2013;121:951-8. [Crossref] [PubMed]
- Steenland MW, Zapata LB, Brahmi D, et al. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception 2013;87:611-24. [Crossref] [PubMed]
- Mohllajee AP, Curtis KM, Peterson HB. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception 2006;73:145-53. [Crossref] [PubMed]
- Toivonen J, Luukkainen T. Progestin-releasing intrauterine devices. Curr Ther Endocrinol Metab 1997;6:281-5. [PubMed]
- Sivin I. Dose- and age-dependent ectopic pregnancy risks with intrauterine contraception. Obstet Gynecol 1991;78:291-8. [PubMed]
- Xiong X, Buekens P, Wollast E. IUD use and the risk of ectopic pregnancy: a meta-analysis of case-control studies. Contraception 1995;52:23-34. [Crossref] [PubMed]
- Randic L, Vlasic S, Matrljan I, et al. Return to fertility after IUD removal for planned pregnancy. Contraception 1985;32:253-9. [Crossref] [PubMed]
- Darney P, Patel A, Rosen K, et al. Safety and efficacy of a single-rod etonogestrel implant (Implanon): results from 11 international clinical trials. Fertil Steril 2009;91:1646-53. [Crossref] [PubMed]
- Hov GG, Skjeldestad FE, Hilstad T. Use of IUD and subsequent fertility—follow-up after participation in a randomized clinical trial. Contraception 2007;75:88-92. [Crossref] [PubMed]
- Andersson K, Batar I, Rybo G. Return to fertility after removal of a levonorgestrel-releasing intrauterine device and Nova-T. Contraception 1992;46:575-84. [Crossref] [PubMed]
- Stoddard AM, Xu H, Madden T, et al. Fertility after intrauterine device removal: a pilot study. Eur J Contracept Reprod Health Care 2015;20:223-30. [Crossref] [PubMed]
- Hubacher D, Lara-Ricalde R, Taylor DJ, et al. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med 2001;345:561-7. [Crossref] [PubMed]
- Modesto W, Dal Ava N, Monteiro I, et al. Body composition and bone mineral density in users of the etonogestrel-releasing contraceptive implant. Arch Gynecol Obstet 2015;292:1387-91. [Crossref] [PubMed]
- Beerthuizen R, van Beek A, Massai R, et al. Bone mineral density during long-term use of the progestagen contraceptive implant Implanon compared to a non-hormonal method of contraception. Hum Reprod 2000;15:118-22. [Crossref] [PubMed]
- Bahamondes MV, Monteiro I, Castro S, et al. Prospective study of the forearm bone mineral density of long-term users of the levonorgestrel-releasing intrauterine system. Hum Reprod 2010;25:1158-64. [Crossref] [PubMed]
- Lopez LM, Chen M, Mullins Long S, et al. Steroidal contraceptives and bone fractures in women: evidence from observational studies. Cochrane Database Syst Rev 2015;CD009849 [PubMed]
- Nexplanon. Food and Drug Administration Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021529s011lbl.pdf. accessed July 14, 2019
- Ramdhan RC, Simonds E, Wilson C, et al. Complications of subcutaneous contraception: a review. Cureus 2018;10:e2132 [PubMed]
- Smith AJB, Harney KF, Singh T, et al. Provider and health system factors associated with usage of long-acting reversible contraception in adolescents. J Pediatr Adolesc Gynecol 2017;30:609-14. [Crossref] [PubMed]
- Hoopes AJ, Teal SB, Akers AY, et al. Low acceptability of certain contraceptive methods among young women. J Pediatr Adolesc Gynecol 2018;31:274-80. [Crossref] [PubMed]
- Secura GM, Madden T, McNicholas C, et al. Provision of no-cost, long-acting contraception and teenage pregnancy N Engl J Med 2014;371:1316-23. Erratum in: N Engl J Med 2014;372:297. [Crossref] [PubMed]
- Mestad R, Secura G, Allsworth JE, et al. Acceptance of long-acting reversible contraceptive methods by adolescent participants in the Contraceptive CHOICE Project. Contraception 2011;84:493-8. [Crossref] [PubMed]
- Cohen R, Sheeder J, Kane M, et al. Factors associated with contraceptive method choice and initiation in adolescents and young women. J Adolesc Health 2017;61:454-60. [Crossref] [PubMed]
- Hoopes AJ, Gilmore K, Cady J, et al. A qualitative study of factors that influence contraceptive choice among adolescent school-based health center patients. J Pediatr Adolesc Gynecol 2016;29:259-64. [Crossref] [PubMed]
- Melo J, Peters M, Teal S, et al. Adolescent and young women's contraceptive decision-making processes: Choosing “the best method for her.” J Pediatr Adolesc Gynecol 2015;28:224-8. [Crossref] [PubMed]
- Coates C, Gordon CM, Simpson T. A qualitative study exploring contraceptive practices and barriers to long-acting reversible contraceptive use in a sample of adolescents living in the Southern United States. J Pediatr Adolesc Gynecol 2018;31:605-9. [Crossref] [PubMed]
- Turner JH. Long-acting reversible contraceptives: addressing adolescent’s barriers to use. Nurse Pract 2019;44:23-30. [Crossref] [PubMed]
- Pritt NM, Norris AH, Berlan ED. Barriers and facilitators to adolescents’ use of long-acting reversible contraceptives. J Pediatr Adolesc Gynecol 2017;30:18-22. [Crossref] [PubMed]
- Clare C, Squire MB, Alvarez K, et al. Barriers to adolescent contraception use and adherence. Int J Adolesc Med Health 2016; [Crossref] [PubMed]
Cite this article as: Bravata G, Patel DR, Omar HA. Long-acting reversible contraception for adolescents. Pediatr Med 2019;2:43.

