
Human papillomavirus infection in adolescents
Introduction
The human papillomavirus (HPV) is the most common sexually-transmitted infection (STI) in the United States and worldwide, affecting the reproductive tract, oropharynx, and anogenital region via skin-skin contact or exchange of bodily fluids. In adolescents and young adults, HPV is associated most commonly with genital warts and changes in epithelial cells that lead to genitourinary cancers in both men and women, and is associated with oral cancers. Transmission occurs through intimate contact and is highly prevalent worldwide. Due to its replication process, affected individuals may present years after initial infection with HPV. Screening for this virus is challenging and not recommended. Vaccination offers primary prevention against HPV infection.
Epidemiology
Prevalence of HPV infections and HPV-associated cancers are highest in non-industrialized countries and disproportionately affect more women than men (1). According to the World Health Organization report, the worldwide prevalence of HPV among women with normal cytological findings is 11.7% (1). The highest prevalence is reported in Sub-Saharan Africa, Latin America and Caribbean, Eastern Europe, and Southeastern Asia (1). Figure 1 depicts affected individuals by race and ethnicity with HPV-associated cancers in the United States as reported by the Centers for Disease Control and Prevention (CDC) (2). Despite decrease in prevalence after the HPV vaccination was introduced, cervical cancer remains one of the leading causes of death in women. In 2012, at least half a million of new cases of cervical cancer was reported globally with higher burden in less non-industrialized countries (3). In the United States, the American Cancer Society (ACS) approximates around 13,000 new cases of invasive cervical cancer diagnosed each year, 30% of which will result in mortality (4). The economic burden of genital HPV has been reported to be about $4 billion yearly (5,6) with an estimated cost of about $4 million for non-cervical-related conditions (7).
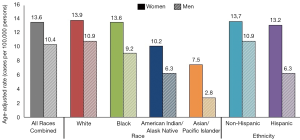
The incidence of HPV infections is highest in women in the 3rd decade of life (1). If infections from high risk HPV types are not cleared, the risk of progression to pre-cancerous and cancerous lesions increases with age. There is no such age-associated risk in men. In sexually actives males, anal HPV infection is very common in men who have sex with men (MSM), especially HIV-positive MSM, the majority of whom also test positive for HPV. Men with ≥3 lifetime partners who have sex with women are also at an increased risk of HPV infection (1).
Human papillomavirus
The family Papillomaviridae is comprised of non-enveloped, double-stranded DNA viruses, that include HPV, and is highly-tissue specific (8,9). Figure 2 illustrates the spherical outer shell and DNA within the HPV (10). Their genome consists of 8,000 base pairs that encode for proteins that promote cell growth (11). HPV primarily affects epithelial cells by invading basal layers of skin. Early genes are expressed that stimulate the host cell DNA polymerase to replicate viral DNA during human cellular division (12,13). As the virus travels through the basal layers of the epidermis, it assembles its structural proteins (12,13).
The HPV is able to evade immune detection and destruction in several ways. Since it does not invade the blood stream, there is no viremic phase and hence, does not induce an immune response (14). It grows very slowly and has low levels of gene expression. Fortunately, for immunocompetent individuals, the infection will clear spontaneously in 3–5 years (14). Those who do not clear HPV are at increased risk of developing cancer, particularly of the genitourinary tract. This is because HPV DNA is coded with oncogenes: E6 and E7. E6 binds and destroys p53 proteins. E7 binds and inactivates retinoblastoma protein (14). Normally, p53 and retinoblastoma proteins act as tumor suppressors, hence their destruction or inactivation lead to unchecked growth of tumor cells (14). HPV DNA becomes integrated in to host DNA cells which increases expression of these oncogenes (14).
High- and low-risk types
The many different strains of HPV are typically characterized by the genomic makeup of the capsid protein, but can be classified further as “high-risk” or “low-risk” based on their ability to predispose cancer in those infected. Figure 3 depicts currently available data by CDC on the estimated number of cancer cases in relation to HPV by sex, cancer type and HPV type (15).
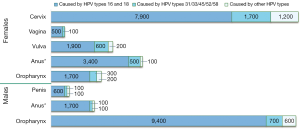
High-risk strains are responsible for 71% of cervical cancers globally. HPV-16 is associated with 60.6% of cervical cancers, while 10.2% results from HPV-18 (1). Carcinomas of the anus (88%), vagina (78%), oropharynx (60%), penis (51%), and vulva (48%) are predominantly caused by type 16 (16-19). Other relatively common high-risk strains include types 31, 33, 35, 45, 52 and 58. Together, all high-risk strains are responsible for 90% of HPV-positive squamous cell carcinomas (1).
Low risk strains are far less associated with cancers, but manifest as benign skin lesions, commonly seen as genital warts (condyloma acuminatum) (1). Types 6 and 11 are the most common low risk strains accounting for up to 90% of anogenital warts (1). Although rare, these strains can become cancerous. Recurrent respiratory papillomatosis (RRP) is a potentially lethal condition in which warts form in the respiratory tract causing airway obstruction that may require surgical intervention (1,20-22).
Disease expression
Disease expression of HPV includes common warts (verruca vulgaris) which occur in about 20% of school-aged children with prevalence declining with age (23). Genital lesions from HPV are acquired from sexual contact, making it the most common STI worldwide. During the years 2013-2014, HPV was reported in nearly half (42.5%) of adults in the United States aged 18–59 years (24,25). Other clinical manifestations include genitourinary pre-cancerous lesions (such as low-grade squamous intraepithelial lesion and high-grade intraepithelial lesion) and cancers [such as vulvar intraepithelial neoplasia, vaginal intraepithelial neoplasia, and cervical intraepithelial neoplasia (CIN)]. The peak age for cervical cancer is from 35 to 44 years (3). Other associated conditions include pre-cancerous lesions and cancers in the rectum and penis, certain oropharyngeal cancers and RRP (26,27).
Health care providers and epidemiologists follow and track these diseases caused by HPV due to the associated risk of development of in situ and invasive squamous cell carcinomas, particularly of the cervix, but also of the vagina, vulva, penis, anus, and oropharynx. Yet, infections from high-risk strains most frequently remain asymptomatic without guaranteed progression to cancer. Approximately 70–90% of HPV-related infections spontaneously resolve within 1–2 years in most healthy immunocompetent individuals (1).
Papanicolau (Pap) smears are done in young adult women to detect early signs of cervical cancer. Persistent HPV lesions can progress towards various stages of precancerous lesions over subsequent months or years. Presence of infection or precancerous lesions should be noted at screening because these risk factors warrant follow-up to monitor for resolution. Lesions that persist require treatment to prevent disease progression to more advanced stages. (1).
Most of these lesions, called CIN will spontaneously resolve, but if found on routine screening, must be staged based on the quality and quantity of dysplasia displayed. From most mild to most severe dysplasia displayed, CIN stages are rated as CIN1, CIN2, and CIN3, including carcinoma in situ. This progression from HPV infection to CIN 3 and invasive carcinoma occurs over the course of many years (1).
Screening recommendations
Pap smear screening for cervical cancer is secondary prevention against cervical cancer (4). Recommendations for screening in the United States are based on guidelines from the ACS (28), American College of Obstetrics and Gynecology (ACOG) (29), and US Preventive Services Task Force (USPSTF) (30). Screening should begin at age 21, regardless of sexual activity, and repeated every 3 years. The recommendations regarding management of abnormal cervical cytology, high-risk HPV testing, or both, depend on age. Screening immunocompetent women younger than the age 21 years and older than 65 years (with prior adequate screening) have lower risk of cervical cancer, hence not recommended. Immunocompromised women (HIV-positive or women on immunosuppressant therapy), require more frequent screening. Thus far, HPV immunization status does not change screening recommendations (31).
Treatment
In healthy immunocompetent adults, most HPV infections resolve spontaneously. For individuals affected by non-cancerous HPV types 6 and 11, majority of cases result in external anogenital warts. The CDC treatment recommendations for these lesions are shown in Table 1 (32). It is imperative to recognize that affected persons with external anogenital warts can have internal lesions as well.
Table 1
| Applied by patient | Administered by the provider |
|---|---|
| Imiquimod 3.75% or 5% cream | Cryotherapy with liquid nitrogen or cryoprobe |
| Podofilox 0.5% solution or gel | Surgical removal (tangential scissor excision, tangential shave excision, curettage, laser or electrosurgery) |
| Sinecatechins 15% ointment | Trichloroacetic acid or bichloroacetic acid 80–90% solution |
CDC, Center for Disease Control and Prevention. Accessed 28 June 2019. Available online: https://www.cdc.gov/std/tg2015/warts.htm
In individuals affected by more than one high-risk type that leads to persistent infections resulting in life threatening conditions, treatment is dependent on the stage of disease. For pre-invasive cervical lesions, curative ablative methods, such as thermal coagulation and freezing, can be successful (33). High risk lesions, such as CIN 2–3 may be removed via cryotherapy, or surgical techniques such as loop electrosurgical excision procedure (LEEP), or cone biopsy for larger/recurrent lesions (33). However, with the availability of an effective vaccine against HPV, prevention is key.
HPV vaccination
HPV vaccination is primary prevention against HPV infection and genitourinary cancers. Currently, there is a United States Food and Drug Administration approved vaccine that protects against nine HPV types: 6, 11, 16, 18, 31, 33, 45, 52 and 58 (34). The 9-valent HPV vaccine is approved for use in males and females, and can be given as early as age 9 (especially for victims of sexual abuse). It is recommended for routine use in children between the ages of 11 and 12 with catch-up recommendations up to age 45 (34).
Due to a better immune response, two doses are recommended when the vaccine series is initiated before age 15. After age 15, three doses are required to ensure protection against HPV. The vaccine is not recommended for pregnant adolescents and adults. It should not be given to anyone who is allergic to any components of the vaccine. Side effects of vaccine administration are generally well tolerated and include injection site reaction (pain, swelling and redness), syncope, lightheadedness, nausea, headache and mild fever (26,34).
Since the introduction of HPV vaccines, there has been a decline in cervical precancerous lesions (35). This indicates that with time, this vaccine - if administered widely—will drastically reduce cervical cancer rates and HPV-associated cancer rates. When compared to similar age-appropriate vaccines in the United States, the rate of completed HPV vaccination series is much lower. For example, rates of vaccination for the Tetanus-diphtheria and acellular Pertussis vaccine and the A/C/Y/W-135 meningococcal vaccine (MCV4/MSPV4) approach 100%, while completed vaccine rates for HPV vaccines is barely 50% for females and even lower for males (36). Figure 4 shows the percentage of adolescents who are current with their HPV vaccinations by state in the United States (37).
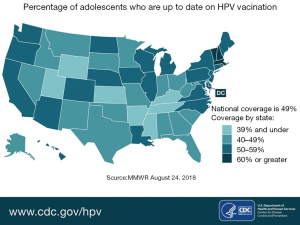
Barriers to HPV vaccination
Some perceived general barriers to immunizing adolescents include: fewer preventive visits overall (annual visits as opposed to every few months for the infant schedule); time constraints; reimbursement concerns; consent; tracking immunization records; and lack of education about the HPV vaccine, such as vaccine safety. Parents are influenced by provider recommendations, news media, other family members and school requirements with respect to likelihood of immunizing their child (38,39).
Hence, health care providers should not underestimate their influence and importance when recommending vaccines. Vaccine messaging from providers should emphasize the safety and efficacy of the HPV vaccine. Specifics regarding HPV acquisition can be tailored based on the age and developmental stage of the adolescent. HPV vaccines should be strongly recommended (26).
A unique aspect of the HPV vaccine is its ability to offer primary prevention against a highly prevalent STI with deleterious outcomes and high economic burden. There have been proposals to allow adolescents to consent for this specific vaccine on their own (as it is designed to prevent an STI and in the United States, many adolescents can consent to STI treatment though they are still considered minors under the law). This consideration would require support from policy makers and health care providers (including public health officials) (40).
Improving HPV vaccination
The conversation should start early which allows timely preparation and understanding from the child and his/her caregivers. Even if the vaccine can be given as old as 45 years, the younger the child initiates the series, the greater the response. The role of the medical practitioner is important when discussing HPV vaccination during clinic visits. One study found providers who make recommendations about the vaccine assuming parents’ readiness to vaccinate had improved initiation of the vaccine among young adolescents (41). The highly effective HPV vaccine can be given as part of the other scheduled adolescent vaccines.
Rand et al. conducted a learning collaborative quality improvement (QI) model in different primary care practices to improve HPV vaccination rates by reducing missed opportunities at all visit types (42). The interventions targeted changes within the office systems, such as identifying a clinic champion, strong recommendations from provider, flagging charts for patients due for vaccines, assessing those in need to be vaccinated during any type of visits (wellness, nurse, acute, chronic, and other visits), and updating office policy on vaccinations. Utilization of the QI model resulted in less missed opportunities to vaccinate, increased overall initiation and completion of series (42).
In a systematic review of interventions done in unvaccinated college students found that increased uptake of the HPV vaccine correlated with encouragement from both peers and medical practitioners (43). Hence, finding creative ways to engage adolescents and families, and to debunk myths about the infection and vaccine, is essential. The recommendations for the HPV vaccination are evolving. It is imperative to emphasize to families that getting immunized prior to exposure is the best way to prevent HPV associated infections.
Conclusions
The HPV is a highly contagious and prevalent infection. Disease burden is high in otherwise healthy young men and women leading to significant consequences. HPV infections also create significant economic burden to society. Although the disease and economic burden of HPV infections is greater in women, men can also be affected by HPV. The available vaccine against HPV is a safe and effective means of primary prevention. Immunogenicity is achieved at better rates when the vaccine is administered to younger adolescents, though the vaccine has been recently approved for use up to age 45 years. Challenges have occurred with administration of this vaccine however, leading to slower uptake when compared to other adolescent specific vaccines. Creative strategies to improve uptake include: utilizing vaccine-only visits and offering immunizations in school-based settings. Given the nature of acquisition of HPV, allowing adolescents to consent to their own immunization is another approach towards slowly but surely eliminating this infection.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Pediatric Medicine for the series “Adolescent Gynecology”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.08.01). The series “Adolescent Gynecology” was commissioned by the editorial office without any funding or sponsorship. MDC served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017-Recommendations. Vaccine 2017;35:5753-5. [Crossref] [PubMed]
- HPV-associated cancers rates by race and ethnicity. Accessed 9 June 2019. Available online: https://www.cdc.gov/cancer/hpv/statistics/race.htm
- WHO: Human papillomavirus (HPV). Accessed 5 June 2019. Available online: https://www.who.int/immunization/diseases/hpv/en/
- American cancer Society. Key Statistics for Cervical Cancer. Accessed 31 Dec 2018. Available online: https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html
- Chesson HW, Blandford JM, Gift TL, et al. The estimated direct medical cost of sexually transmitted diseases among American youth, 2000. Perspect Sex Reprod Health 2004;36:11-9. [Crossref] [PubMed]
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005;23:1107-22. [Crossref] [PubMed]
- Hu D, Goldie SJ. The economic burden of non-cervical human papillomavirus disease in eth United States. Am J Obstet Gynecol 2008;198:500.e1-7. [Crossref] [PubMed]
- Bzhalava D, Guan P, Franceschi S, et al. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 2013;445:224-31. [Crossref] [PubMed]
- Bonnez W, Reichman RC. Papilomaviruses. In: Mandell GL, Bennett JE, Dolin R. editors. Principles and practice of infectious diseases. New York: Churchill Livingstone Inc., 2000:2035-50.
- HPV virus. Accessed 9 June 2019. Available online: https://twitter.com/CDCSTD/status/508692453835042817
- Favre M. Structural polypeptides of rabbit, bovine, and human papillomaviruses. J Virol 1975;15:1239-47. [PubMed]
- Flores ER, Lambert PF. Evidence for a switch in the mode of human papillomavirus type 15 DNA replication during the viral life cycle. J Virol 1997;71:7167-79. [PubMed]
- Flores ER, Allen-Hoffman BL, Lee D, et al. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology 1999;262:344-54. [Crossref] [PubMed]
- Kanodia S, Fahey LM, Kast WM. Mechanisms used by human papillomavirus to escape the host immune response. Curr Cancer Drug Targets 2007;7:79-89. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Cancers associated with human papillomavirus, United States—2011–2015 USCS data brief, no. 4. Atlanta, GA: Centers for Disease Control and Prevention 2018. Accessed 18 June 2019. Available online: https://www.cdc.gov/cancer/hpv/pdf/USCS-DataBrief-No4-August2018-508.pdf
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006;24 Suppl 3:S3/11-25.
- Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: Implications for current 9-valent HPV vaccines. J Natl Cancer Inst 2015;107:djv086 [Crossref] [PubMed]
- Clifford GM, Rana RK, Franceschi S, et al. Human Papillomavirus genotype distribution in low-grade cervical lesions: Comparison by geographic regions and with cervical cancer. Cancer Epidemiol Biomarkers Prev 2005;14:1157-64. [Crossref] [PubMed]
- Porras C, Rodriguez AC, Hildesheim A, et al. Human Papillomavirus types by age in cervical cancer precursors: Predominance of human papillomavirus 16 in young women. Cancer Epidemiol Biomarkers Prev 2009;18:863-5. [Crossref] [PubMed]
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A 1983;80:560-3. [Crossref] [PubMed]
- Wiley D, Mansongsong E. Human papillomavirus: the burden of infection. Obstet Gynecol Surv 2006;61:S3-14. [Crossref] [PubMed]
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4:Burden and management on non-cancerous HPV-related conditions:HPV-6/11 disease. Vaccine 2006;24:S3/35-41.
- Kilkenny M, Merlin K, Young R, et al. The prevalence of common skin conditions in Australian school students:1. Common, plane and plantar viral warts. Br J Dermatol 1998;138:840-5. [Crossref] [PubMed]
- Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sex Transm Dis 2013;40:187-93. [Crossref] [PubMed]
- McQuillan G, Krucszon-Moran D, Markowitz LE, et al. Prevalence of HPV in adults aged 18-69: United States, 2011-2014. NCHS Data Brief 2017;1-8. [PubMed]
- Human Papillomavirus (HPV). Genital HPV Infection Fact Sheet. Centers for Disease Control and Surveillance. Accessed 17 Dec 2018. Available online: https://www.cdc.gov/std/hpv/stdfact-hpv.htm
- Recurrent Respiratory Papillomatosis or Laryngeal Papillomatosis. National Institute on Deafness and Other Communication Disorders. Accessed 18 Dec 2018. Available online: https://www.nidcd.nih.gov/health/recurrent-respiratory-papillomatosis
- American Cancer Society. The American Cancer Society Guidelines for the prevention and early detection of cervical cancer. Accessed 18 June 2019. Available online: https://www.cancer.org/cancer/cervical-cancer/prevention-and-early-detection/cervical-cancer-screening-guidelines.html
- American College of Obstetricians and Gynecologists. Cervical cancer screening and prevention. Practice Bulletin No. 168. Obstet Gynecol 2016;128:e111-30. [Crossref] [PubMed]
- Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force Recommendation Statement. US Preventive Services Task Force. JAMA 2018;320:674-86. [Crossref] [PubMed]
- Workowski KA, Bolan GACenters for Disease Control and Prevention. Sexually transmitted guidelines, 2015. MMWR Recomm Rep 2015;64:1-137. [PubMed]
- CDC. 2015 Sexually transmitted diseases treatment guidelines. Anogenital Warts. Accessed 28 June 2019. Available online: https://www.cdc.gov/std/tg2015/warts.htm
- Castle PE, Murokora D, Perez C, et al. Treatment of cervical intraepithelial lesions. Int J Gynaecol Obstet 2017;138:20-5. [Crossref] [PubMed]
- Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination - Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2016;65:1405-8. [Crossref] [PubMed]
- Watson M, Soman A, Flagg EW, et al. Surveillance of high-grade cervical cancer precursors (CIN III/AIS) in four population-based cancer registries, United States, 2009–2012. Prev Med 2017;103:60-5. [Crossref] [PubMed]
- Walker TY, Elam-Evans LD, Yankey D, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years - United States, 2017. MMWR Morb Mortal Wkly Rep 2018;67:909-17. [Crossref] [PubMed]
- Percentage of adolescents who up to date on HPV vaccination. Accessed 9 June 2019. Available online: https://www.cdc.gov/hpv/infographics/vacc-coverage.jpg
- Holman DM, Benard V, Roland KB, et al. Barriers to human papillomavirus vaccination among U.S. adolescents: A systematic review of the literature. JAMA Pediatrics 2014;168:76-82. [Crossref] [PubMed]
- Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11- to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev 2009;18:2325-32. [Crossref] [PubMed]
- English A, Ford C, Kahn JA, et al. Adolescent consent for vaccination: A position paper of the Society for Adolescent Health and Medicine. Journal of Adolescent Health 2013;53:550-53. [Crossref] [PubMed]
- Brewer NT, Hall ME, Malo TL, et al. Announcements versus conversations to improve HPV vaccination coverage: A randomized trial. Pediatrics 2017; [Crossref] [PubMed]
- Rand CM, Tyrrell H, Wallace-Brodeur R, et al. A learning collaborative model to improve human papillomavirus vaccination rates in primary care. Acad Pediatr 2018;18:S46-52. [Crossref] [PubMed]
- Barnard M, Cole AC, Ward L, et al. Interventions to increase uptake of the human papillomavirus in unvaccinated college students: A systematic literature review. Prev Med Rep 2019;14:100884 [Crossref] [PubMed]
Cite this article as: Holder N, Ahmed N, Cabral MD. Human papillomavirus infection in adolescents. Pediatr Med 2019;2:46.

