Risk factors for necrotizing enterocolitis associated mortality
Introduction
Necrotizing enterocolitis (NEC) is one of the most serious gastrointestinal diseases in neonates, especially in preterm babies. NEC associated mortality is one of the major causes of death in preterm infants (1). The incidence of NEC is high. In the United States and Canada, the morbidity of NEC in very low BW (VLBW) is about 7%, and the mortality is as high as 20–30% (2). NEC was associated with approximately 10% of neonatal deaths in the United States, and it was the third etiology of deaths in neonatal intensive care unit (NICU) (1). Although more and more extremely preterm babies survive, the number of deaths attributed to NEC is increasing (3).
Unfortunately, the pathophysiology of NEC is not exactly clear. Multi-factorial causes such as intestinal immaturity, formula feeding, hypoxia-ischemia, abnormal colonization of intestinal flora, and outbreak of intestinal unregulated immune response, lead to intestinal necrosis and multiple organs dysfunction (4-6). Low BW and prematurity were the most identified risk factors related to develop NEC (7).
The purpose of this research was to identify risk factors for NEC associated mortality in our NICU during 2015–2017, and allowing for the development of targeted strategies to avoid death.
Methods
Selection of study population
Ethical approval was obtained for this retrospective case-control study. One hundred and sixty-three infants with confirmed NEC were included in our NICU during January 2015 to December 2017. Confirmed NEC was newborn diagnosed with NEC stage II and stage III by Bell’s criteria and modified by Walsh and Kliegman (8,9).
Data collection
According to different short-term outcomes, they were divided into survival group and death group. Data in different groups included demographic, clinical, hematological, radiological, and operative details was compared for risk factors predicting mortality.
Statistical analysis
Statistical analysis was performed with SPSS-22.0. Predictive factors were identified using Student t-tests, or chi-square test as appropriate. The factors that were significantly different between the two groups in bivariate analyses were adjusted in multivariable regression models. The difference was considered statistically significant for P value <0.05.
Results
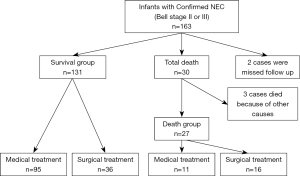
In total, 163 cases diagnosed confirmed NEC were included during the study period. Five cases were excluded, because two were failed to follow up and three died of other causes. Of the 158 remaining infants, 52 (32.9%) infants required surgical intervention. According to different short-term outcomes they were divided into survival group (131 cases) and death group (27 cases) (Figure 1).
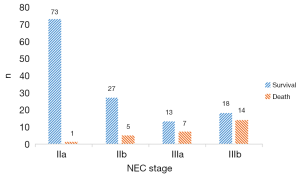
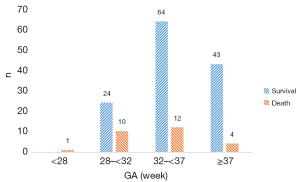
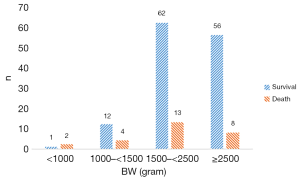
There were 74 stage IIa cases, 32 stage IIb cases, 20 stage IIIa cases, 32 stage IIIb cases (Figure 2). The overall mortality rate was 17.1%. GA and BW distribution in survival group and death group is showed in Figure 3 and Figure 4.
Demographic and clinical data of NEC infants were collected and analyzed. In death group GA was smaller, BW was lighter, 5-min Apgar score was lower significantly than survival group. And the rate of respiratory support or the rate of caffeine treated was significantly higher in the death group. There was no significant difference in gender, age of onset, fetal distress, 1-min Apgar score, 10-min Apgar score, oral probiotics, blood transfusion between the two groups (Table 1).
Table 1
| Baseline characteristics | Survival group (n=131) | Death group (n=27) | P |
|---|---|---|---|
| Gender, male, n (%) | 67 (51.1) | 14 (51.9) | 0.947 |
| GA (wk) | 35.31±3.32 | 33.36±3.71 | 0.016 |
| BW (g) | 2396.87±750.59 | 1957.04±701.65 | 0.006 |
| Age of onset (d) | 8.27±6.40 | 10.94±7.27 | 0.084 |
| Fetal distress, n (%) | 16 (12.2) | 4 (14.8) | 0.711 |
| Apgar score | |||
| 1-min | 8.40±1.83 | 7.56±2.06 | 0.057 |
| 5-min | 9.17±1.33 | 8.52±1.89 | 0.034 |
| 10-min | 9.49±0.89 | 9.19±1.08 | 0.179 |
| Treatment before NEC onset, n (%) | |||
| Respiratory support | 27 (20.6) | 13 (48.1) | 0.003 |
| Oral probiotics | 28 (21.4) | 6 (22.2) | 0.922 |
| Blood transfusion | 17 (13.0) | 4 (14.8) | 0.798 |
| Caffeine | 19 (14.5) | 9 (33.3) | 0.020 |
NEC, necrotizing enterocolitis; GA, gestational age; BW, birth weight.
In our study, there were statistically significant differences in the two groups about thrombocytopenia (platelet count <100×109/L), leukopenia (white blood cell count <5×109/L), highest CRP value, and Duke abdominal assessment scale (DAAS) (10). But there was no significant difference in onset CRP value (Table 2).
Table 2
| Hematological, radiological results | Survival group (n=131) | Death group (n=27) | P |
|---|---|---|---|
| Thrombocytopenia, n (%) | 16 (12.2) | 13 (48.1) | 0.000 |
| Leukopenia, n (%) | 44 (33.6) | 18 (66.7) | 0.001 |
| Onset CRP value (mg/L) | 32.17±37.26 | 40.51±38.29 | 0.308 |
| Highest CRP value (mg/L) | 48.71±47.95 | 74.89±60.88 | 0.043 |
| DAAS | 7.19±2.149 | 8.33±1.981 | 0.010 |
The Duke abdominal assessment scale (DAAS) (
Fifty-two infants required surgical intervention. Three infants required primary peritoneal drainage, and the range of intestinal necrosis is not clear. One infant required surgery because of frequently vomiting and repeated abdominal distension in the recovered period, but there was no intestinal necrosis found in the operation, and its final diagnosis is NEC complicated with aganglionosis. Excluding above 4 infants, data from 48 cases at laparotomy were analyzed. We found that intestinal perforation or not was not associated with mortality in surgical patients, but identified a greater range of intestinal necrosis was an increased risk of death (Table 3).
Table 3
| Findings at laparotomy | Survival group (n=35), n (%) | Death group (n=13), n (%) | P |
|---|---|---|---|
| Perforation | 18 (51.4) | 4 (30.8) | 0.202 |
| No peroration | 17 (48.6) | 9 (69.2) | |
| The necrotic range of intestine | |||
| Focal | 15 (42.9) | 1 (7.7) | 0.021 |
| Multifocal | 15 (42.9) | 6 (46.2) | |
| Pan intestinal | 5 (14.3) | 6 (46.2) |
NEC, necrotizing enterocolitis.
Since the range of intestinal necrosis was unknown in medically managed infants, it was excluded from the regression models. Other factors that were significantly different between the survival and death groups were adjusted in multivariable regression models. Multivariable logistic regression analysis was performed for GA, BW, 5-min Apgar score, respiratory support, caffeine, leukopenia, thrombocytopenia, the highest CRP value, DAAS. It showed that thrombocytopenia remained a risk factor statistically significantly for NEC associated mortality (Table 4).
Table 4
| Related factors | B | Standard error | P | Odds ratio | 95% CI |
|---|---|---|---|---|---|
| GA | 0.145 | 0.152 | 0.340 | 1.156 | 0.858–1.559 |
| BW | −0.001 | 0.001 | 0.274 | 0.999 | 0.998–1.001 |
| 5-min Apgar score | −0.146 | 0.164 | 0.374 | 0.864 | 0.626–1.192 |
| Respiratory support | 0.469 | 0.680 | 0.490 | 1.599 | 0.422–6.062 |
| Caffeine | 0.207 | 0.764 | 0.786 | 1.230 | 0.275–5.501 |
| Leukopenia | 0.475 | 0.587 | 0.419 | 1.607 | 0.508–5.082 |
| Thrombocytopenia | 1.680 | 0.584 | 0.004 | 5.365 | 1.709–16.840 |
| Highest CRP value | −0.002 | 0.005 | 0.676 | 0.998 | 0.988–1.008 |
| DAAS | 0.291 | 0.175 | 0.097 | 1.337 | 0.949–1.885 |
95% CI, 95% confidence interval; GA, gestational age; BW, birth weight; CRP, C-reaction protein; DAAS, Duke abdominal assessment scale.
Discussion
NEC is one of the most devastating diseases in neonates, it is a major cause of death in preterm infants. Onset of NEC is insidious. Initially, clinical presentations are nonspecific such as temperature instability, apnea, lethargy, feeding intolerance, increased residual gastric volumes, emesis, bloody stools, and mild abdominal distension. However, these are common in very preterm infants in NICU. NEC can progress rapidly to shock and multiple organ dysfunction in a short time.
Preterm birth is a recognized risk factor of NEC. Premature intestine has immature motility, digestion, absorption, immune defenses, barrier function, and is easily injured (11). Preterm infants with formula feeding, hypoxia-ischemia, an abnormal microbial intestinal colonization, have an excessive inflammatory response leads to intestinal necrosis. In recent years, the global incidence of preterm birth is increasing (3,12). In China, the number of pregnancy with advanced maternal age, or multiple pregnancy with assisted reproduction is increasing, therefore, the incidence of preterm birth is rising too. In this study, average GA of infants with confirmed NEC is 34.98±3.46 w, and average BW is 2,321.71±758.69 g. Compared with developed countries, the average GA of NEC infants is greater, the average BW of NEC infants is heavier. There are several reasons. First, there has less very preterm infant or VLBW hospitalized in our unit. Second, the rate of human milk feeding is lower, and we do not have human milk bank.
The rate of respiratory support or the rate of caffeine treatment was significantly higher in the death group. This may associate with GA or BW. Infants may easily have neonatal respiratory distress syndrome (NRDS) or apnea of prematurity (AOP), if they have a smaller GA or lighter BW. Duro (13) found that mechanical ventilation is a risk factor of gastrointestinal failure of children with NEC in their research. Five-min Apgar score reflects the effects of resuscitation in infants, intestinal ischemia may be more severe when the score is lower.
In the systemic analysis completed by Song et al. (14), they found that NEC patients may have thrombocytopenia, disseminated intravascular coagulation (DIC), neutrophilia, neutropenia, hemolytic anemia and some other complications. All the complications participate intestinal injury directly or indirectly, they can predict some important clues. Our study also revealed that the mortality rises with the complications as leukopenia (WBC <5×109/L), thrombocytopenia (PLT <100×109/L), and high peak value of C-reaction protein (CRP) during the course of NEC. Pourcyrous et al. (15) found that more patients lead surgical intervention and worse outcome if the CRP keeps elevating. In our study, thrombocytopenia is the independent risk factor of death in NEC patients, this result indicates that severe infection not only induces NEC, but also associated with an increased risk of death.
According to the diagnosis of NEC, we have been employing the modified Bell’s staging since 1986, which is based on the systemic symptoms, abdominal signs, and radiologic findings. From these aspects, we can also classify all the NEC patients into three stages (I, II and III), all of which could be further divided into grade A and B. As widely known, there is a close relationship between staging and prognosis. With respect to the stage IIIA and IIIB, the major difference between them is the existence of pneumoperitoneum.
Intestinal perforation or not in patients with NEC IIIA and IIIB who received exploratory laparotomy was not associated with mortality in our study, but the necrosis range of the intestinal tract is the risk factor of death, this is similar to the report by Thyoka (16). Thus, a highly sensitive and specific biomarker may be the key to modify the diagnostic criteria of NEC (2), which can improve prognosis and reduce mortality. So the researches about NEC inflammatory markers have kept popular in recent years.
In our study, the mortality of the patients who received surgical therapy is 30.8% (16/52), which is nearly the same as 38.8% (66/170) reported by Thyoka (16), both are significantly different from the 50% mortality post-surgical treatment reported by Kastenberg (17). There might be two reasons. Firstly, more than 85% of NEC cases in developed countries are extremely preterm or VLBW infants (BW <1,500 g, GA <32 w) (18), they were intolerant the disease and surgical procedure. Secondly, our study is limited in short term follow up, while the patients may have some complications such as intestinal stricture, short-bowel syndrome, recurrent NEC, cholestasis, neurodevelopmental impairment, extrauterine growth retardation, all of which will contribute to the negative prognosis. In our unit, some patients had reached the indicates of surgical intervention, but their parents refused operation because of religion or others. General and peritoneal conditions of these patients might deteriorate quickly. Finally, they missed the best opportunities for laparotomy. These tragedies remind us that we should communicate with the parents reasonably to avoid unnecessary death.
Conclusions
In our study, thrombocytopenia in NEC infants was associated with an increased risk of mortality. In surgical patients, greater range of intestine necrosis was associated with an increased risk of mortality, but intestinal perforation or no perforation was not related to death. NEC is a devastating disease and a leading cause of death, it is not so feasible to seek some more revolutionary therapies against NEC in the short term. Thus, we need to pay more attention to the prevention of NEC, take actions to reduce preterm delivery and encourage breast milk feeding (19) to the preterm infants. In summary, preventive measures, early diagnosis and treatment of NEC are important to reduce morbidity and mortality, and improve long term outcome for preterm babies.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.11.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was obtained for this retrospective case-control study was approved by the Medical Ethics Committee of Chengdu Women and Children’s Central Hospital (No. 2018-002) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jacob J, Kamitsuka M, Clark RH, Kelleher AS, Spitzer AR. Etiologies of NICU deaths. Pediatrics 2015;135:e59-65. [Crossref] [PubMed]
- Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255-64. [Crossref] [PubMed]
- Patel RM, Kandefer S, Walsh MC, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med 2015;372:331-40. [Crossref] [PubMed]
- Koletzko B, Poindexter B, Uauy R. editors. Nutritional Care of Preterm Infants: Scientific Basis and Practical Guidelines. 2014. Karger: Basel, Switzerland.
- Tanner SM, Berryhill TF, Ellenburg JL, et al. Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. Am J Pathol 2015;185:4-16. [Crossref] [PubMed]
- Berkhout DJC, Klaassen P, Niemarkt HJ, et al. Risk Factors for Necrotizing Enterocolitis: A Prospective Multicenter Case-Control Study. Neonatology 2018;114:277-284. [Crossref] [PubMed]
- Samuels N, van de Graaf RA, de Jonge RCJ, et al. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr 2017;17:105. [Crossref] [PubMed]
- Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1-7. [Crossref] [PubMed]
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179-201. [Crossref] [PubMed]
- Coursey CA, Hollingsworth CL, Wriston C, et al. Radiographic predictors of disease severity in neonates and infants with necrotizing enterocolitis. AJR Am J Roentgenol 2009;193:1408-13. [Crossref] [PubMed]
- Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotising enterocolitis. Semin Fetal Neonatal Med 2006;11:369-77. [Crossref] [PubMed]
- March of Dimes; Partnership for Maternal, Newborn Child Health; Save the Children; World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth. World Health Organization: Geneva, Switzerland, 2012.
- Duro D, Kalish LA, Johnston P, et al. Risk factors for intestinal failure in infants with necrotizing enterocolitis: a Glaser Pediatric Research Network study. J Pediatr 2010;157:203-8.e1. [Crossref] [PubMed]
- Song R, Subbarao GC, Maheshwari A. Haematological abnormalities in neonatal necrotizing enterocolitis. J Matern Fetal Neonatal Med 2012;25:22-5. [Crossref] [PubMed]
- Pourcyrous M, Korones SB, Yang W, et al. C-reactive protein in the diagnosis, management, and prognosis of neonatal necrotizing enterocolitis. Pediatrics 2005;116:1064-9. [Crossref] [PubMed]
- Thyoka M, de Coppi P, Eaton S, et al. Advanced necrotizing enterocolitis part 1: mortality. Eur J Pediatr Surg 2012;22:8-12. [Crossref] [PubMed]
- Kastenberg ZJ, Sylvester KG. The surgical management of necrotizing enterocolitis. Clin Perinatol 2013;40:135-48. [Crossref] [PubMed]
- Thompson AM, Bizzarro MJ. Necrotizing enterocolitis in newborns: pathogenesis, prevention and management. Drugs 2008;68:1227-38. [Crossref] [PubMed]
- Maffei D, Schanler RJ. Human milk is the feeding strategy to prevent necrotizing enterocolitis!. Semin Perinatol 2017;41:36-40. [Crossref] [PubMed]
Cite this article as: Fu Y, Ju R, Yue G, Xiao T, Zhang X, Gao S, Liu Y, Hu X. Risk factors for necrotizing enterocolitis associated mortality. Pediatr Med 2020;3:2.