A narrative review of retinoblastoma and recent advances in its management
Introduction
Retinoblastoma (RB) is the most common malignant tumor of the eye in childhood. RB begins in the retina and, if untreated, can spread to the lymph nodes, bones, or bone marrow, and rarely, it involves the central nervous system (CNS). RB is caused by mutation of the RB1 gene, which is a tumor-suppressor gene (1-3). There is no racial or gender predisposition for RB. The treatment of RB depends on the anatomical location, laterality (unilateral or bilateral), the number of lesions, size, evidence of subretinal fluid, presence of localized or diffuse vitreous seeding, risks for secondary tumors, presence of metastases, systemic involvement and visual prognosis. The keys to a successful outcome depend on early detection, multidisciplinary, individualised and contemporary care. The objective of this narrative review is to discuss a general overview of RB and broad principles and recent advances in its management. We will discuss this review under the following headings: epidemiology, genetics, clinical manifestations, diagnosis, management, prognosis and conclusion/summary.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/pm-20-79).
Methods
Information used to write this review was collected from the following sources:
- PubMed/Medline/Google Scholar search 1970–July 2020;
- Hand searches of the references of retrieved literature;
- Discussions with experts in the field.
Epidemiology
The incidence of RB varies in one case per 15,000–20,000 livebirths in the west, resulting in approximately 9,000 new cases every year (4). In the majority of cases, it is unilateral and is bilateral in about 30–40% of cases. Of the unilateral cases, 15% are hereditary. All bilateral and multifocal unilateral forms are hereditary. The average age at diagnosis of RB is 18 months with the non-hereditary cases being diagnosed at around 24 months and hereditary ones before 12 months of age (5).
The diagnosis of RB beyond the age of 6 years is rare. Sporadic bilateral RB has been associated with advanced parental age (6,7). In 0.05% of patients, RB has been associated with multiple congenital anomalies.
Developing regions having a large population show a high disease burden (4). The mortality is 40–70% in countries which have the highest prevalence, compared to 3–5% in developed nations (6). Causes of high mortality in these countries includes delay in diagnosis, malnutrition, advanced disease at presentation, lack of access to advanced health care, and lack of standard management protocols for RB (8).
Genetics of RB
RB1 is the tumor suppressor gene that is known to be associated with RB. It is associated with both germline and somatic mutations in this gene. The encoded protein functions as a cell cycle regulator. Numerous point mutations, promoter methylations, and deletions can impair the function of the protein (9). Malignant transformation probably results from both genetic and epigenetic modifications (10).
Other genes and micro RNA (ribonucleic acid) also have an altered level of expression in RB. Sometimes in a small proportion of unilateral RB presenting at young ages, the oncogene MYCN is amplified at high levels.
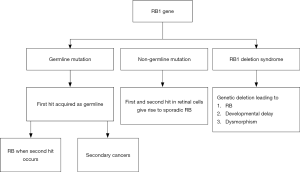
The RB1 gene is inherited in an autosomal dominant manner. Ninety percent of persons with a germline RB1 mutation will develop RB (60% multifocal, 30% unifocal), the remaining 10% remain as unaffected carriers. This can be explained by the Knudson two-hit hypothesis. In heritable RB, the first mutation is in the germ cell, and this ‘first hit’ is carried in every cell in the body, making them susceptible to RB and other cancers. The ‘second hit’ occurring in the retinal cells during retinal development causes RB. In non-heritable RB, both hits occur in the retinal cell, and thus the mutation is confined to the retinal tumor tissue (3).
Heritable RB constitutes 30–40% of all RB and the non-heritable form constitutes 60–70%. Most children with a heritable RB get it as a de-novo mutation and only 10% will have a positive family history. Germline RB usually presents early in life. Of children with unilateral disease, germline mutations are present in 15% (11) (Figure 1).
Genetic counselling in RB
Genetic diagnosis should be performed for all children with RB as a germline variety of RB, needs careful follow-up and evaluation. Parents of children with RB should undergo genetic testing before further pregnancies and so should the siblings so that their risk is ascertained. In the case of bilateral RB, it is necessary to have a genetic diagnosis so that the same mutation can be targeted for detection in the sibling of the proband (12).
After counseling the peripheral blood is collected and the DNA screened for RB1 mutation. In a bilaterally affected proband, there is a 97% probability of finding a mutation, whereas in 3% it may not be detected (due to the presence of low level of mosaicism). For unilateral tumors, the tumor tissue, if available, should be tested for both mutations. If one of the two mutations is identified in blood DNA, the germline status is confirmed; if not, the risk of germline disease is reduced to <1%. If no RB1 mutation is found in the tumor tissue the MYCN gene abnormalities should be looked for (13).
No examination under anesthesia (EUA) is required for the unaffected eye with only clinical follow up in the absence of a germline mutation. The presence of a germline mutation renders the child susceptible to recurrent tumors and also to secondary tumors. The child needs frequent EUA up to 5 y of age and annual surveillance (14).
Antenatal-diagnosis
Based on the pretest probability (15) (Table 1), appropriate counseling can be provided to a couple who has a child with RB and is planning further pregnancies/or if the mother is already pregnant. Various techniques, including amniocentesis, chorionic villus sampling (CVS), and preimplantation biopsy, can be used to obtain a fetal sample and determine whether a fetus is a carrier or not.
Table 1
| Status of genetic test | Bilateral RB | Unilateral RB (blood only if tumor tissue not available OR if both in blood and tumor tissue) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Proband | Offspring | Unaffected parent | Sibling | Proband | Offspring | Unaffected parent | Sibling | ||
| No genetic test done | 100 | 50 | 5 | 2.5 | 15 | 7.5 | 0.75 | 0.38 | |
| Positive test for a known mutation | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Negative blood genetic testing | NA | 0.006 | 0.2 | 0.006 | NA | 0.006 | 0.2 | 0.006 | |
| Mutation not found in blood | 100 | 50 | 5 | 2.5 | 0.45 | 0.22 | 0.02 | 0.01 | |
Clinical presentation
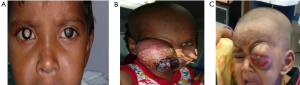
RB is usually diagnosed at about 18 months, with 95% of children being diagnosed by 5 years. Leucocoria is the most common presenting symptom of RB, followed by strabismus, painful blind eye, and loss of vision (16). The common presenting signs and symptoms are depicted in Table 2. RB’s atypical manifestations are pseudohypopyon, spontaneous hyphema, vitreous hemorrhage, phthisis bulbi, pre-septal or orbital cellulitis, iris-rubeosis, buphthalmia, and exophthalmia. The presence of multiple primary tumors or bilateral RB supports the diagnosis of an inherited RB (Figure 2).
Table 2
| Symptoms | % |
|---|---|
| Leucocoria (white pupillary reflex) | 56 |
| Strabismus | 20 |
| Red painful eye | 7 |
| Poor vision | 5 |
| Asymptomatic | 3 |
| Orbital cellulitis | 3 |
| Phthisis bulbi, proptosis (fungating mass) | 3 |
| Unilateral mydriasis | 2 |
| Heterochromia iridis | 1 |
| Hyphema | 1 |
Without indirect ophthalmoscopy, initial lesions are likely to be missed. The patient may present with strabismus or with reduced visual acuity, if the tumor involves the macula (17). A patient with a suspicious RB is best evaluated EUA for the detailed fundus examination. There are three types in which the RB can grow (Figure 3):
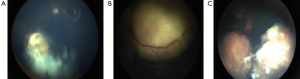
- Endophytic: the tumor grows into the vitreous cavity. A yellow-white mass progressively fills the entire vitreous cavity and vitreous seeding occurs. The retinal vessels are not seen on the surface.
- Exophytic: the tumor grows towards the subretinal space. Retinal detachment occurs and vessels are seen over the tumor.
- Diffuse infiltrating tumor: this causes a placoid thickness of the retina and is seen in older children with a delay in the diagnosis of such cases (18).
Few patients present with advanced disease with proptosis, optic nerve extension, orbital extension, and systemic metastasis. RB can spread through the optic nerve when the lamina cribrosa is breached. Orbital extension of RB may present with proptosis and is most likely to occur at the scleral emissary veins site.
Diagnosis
Differential diagnosis
Coat’s disease is the most important differential diagnosis of RB. Coat’s disease is a unilateral retinal disease with telangiectasia and subretinal exudates. The other differential diagnoses have been listed in Table 3. An invasive form of RB which is usually not calcified is very difficult to differentiate from advanced forms of coat’s disease and Toxocara Canis eye infection due to its atypical clinical and radiological features (19).
Table 3
| Non-infectious causes |
| Coats’ disease |
| Persistent fetal vasculature (PFV) or |
| Persistent hyperplastic primary vitreous (PHPV) |
| Familial exudative vitreoretinopathy |
| Congenital cataract |
| Coloboma |
| Juvenile xanthogranuloma |
| Norrie’s disease |
| Astrocytic and combined hamartoma |
| Medulloepithelioma |
| X-linked retinoschisis |
| Incontinentia pigmenti |
| Vitreous hemorrhage |
| Retinal detachment |
| Retinopathy of prematurity |
| Infectious |
| Endogenous endophthalmitis |
| Toxocariasis |
Investigations
The diagnosis of RB is clinical and additional tests like ultrasonography (USG), computed tomography (CT), and magnetic resonance imaging (MRI) aid in the documentation of the disease and differentiation from pseudo-RB (Table 3) (20,21).
USG B-scan shows a rounded or irregular intraocular mass with high internal reflectivity representing typical intralesional calcification. CT typically shows an intraocular mass with a higher density than the vitreous body, calcified in 90% of cases, and moderately enhanced after iodine contrast agent injection. MRI is the imaging of choice and detects optic nerve invasion, an extension to the anterior chamber, and orbital fat. MRI may be useful for distinguishing RB from pseudo-RB conditions such as coats disease or eye malformations and to diagnose rare cases of trilateral RB (third tumor in the pineal gland or parasellar region).
A EUA of the ocular fundus is confirmatory for diagnosis. The lesion appears as a white tumor with angiomatous dilatation of the vessels. Retinal detachment may also be observed in exophytic forms.
The intraocular pressure (IOP) is measured and the anterior segment is examined for neovascularization, pseudohypopyon, hyphema, and signs of inflammation. Bilateral fundus examination with 360-degree scleral depression is mandatory in all cases. Direct visualization of the tumor by an indirect ophthalmoscope is diagnostic of RB in more than 90% of the cases. It is one of the tumors where treatment can be started without a histopathological confirmation.
The CSF examination, bone scan, and bone marrow aspiration and biopsy are done to rule out systemic involvement when there are features of extraocular spread (20). Imaging with a bone scan or positron emission tomography (PET)-CT is also recommended for metastasis.
Staging of RB
Various classifications for RB have been introduced for predicting vision, globe salvage, and survival. RB that has not spread outside the globe is called intraocular-RB (IORB) and if it has spread to the extra-ocular structures it is termed as extraocular-RB (EORB).The Reese-Ellsworth classification for IORB (22) has been shown in Table 4. The American Joint Committee on Cancer (AJCC) proposed a clinical and pathologic staging for RB. For bilateral RB, each eye is staged separately. Histologic verification of the disease in an enucleated eye is required. The extent of retinal involvement is indicated as a percentage of the total retinal area. A revised staging classification called the International Classification of Retinoblastoma (ICRB) was adopted in 2003 (23-25) (Table 5, Figure 4). The staging of RB has been shown in Table 6.
Table 4
| Group | Findings on EUA |
|---|---|
| Group I: very favorable | (a) Solitary tumor, less than 4 disc diameters in size, at or behind the equator |
| (b) Multiple tumors, none over 4 disc diameters in size, all at or behind the equator | |
| Group II: favorable | (a) Solitary tumor, 4 to 10 disc diameters in size, at or behind the equator |
| (b) Multiple tumors, 4 to 10 disc diameters in size, behind the equator | |
| Group III: doubtful | (a) Any lesion anterior to the equator |
| (b) Solitary tumors larger than 10 disc diameters behind the equator | |
| Group IV: unfavorable | (a) Multiple tumors, some larger than 10 disc diameters |
| (b) Any lesion extending anteriorly to the ora serrata | |
| Group V: very unfavorable | (a) Massive tumors involving over half the retina |
| (b) Vitreous seeding |
*, refers to chances of salvaging the affected eye and not systemic prognosis.
Table 5
| Group | Subgroup | Quick reference | Specific features |
|---|---|---|---|
| A | A | Small tumor | RB ≤3 mm in size* |
| B | B | Larger tumor | RB>3 mm in size* or |
| Macula | • Macular RB location (≤3 mm to foveola) | ||
| Juxtapapillary | •Juxtapapillary RB location (≤1.5 mm to disc) | ||
| Subretinal fluid | • Clear subretinal fluid (≤ 3 mm from margin) | ||
| C | Focal seeds | RB with | |
| C1 | • Subretinal seeds (≤3 mm from RB) | ||
| C2 | • Vitreous seeds (≤3 mm from RB) | ||
| C3 | • Both subretinal and vitreous seeds (≤3 mm from RB) | ||
| D | Diffuse seeds | RB with | |
| D1 | • Subretinal seeds (>3 mm from RB) | ||
| D2 | • Vitreous seeds (>3 mm from RB) | ||
| D3 | • Both subretinal and vitreous seeds (>3 mm from RB) | ||
| E | E | Extensive RB | Extensive RB occupying >50% globe or |
| • Neovascular glaucoma | |||
| • Opaque media from hemorrhage in anterior chamber, vitreous or subretinal space | |||
| • Invasion of postlaminar optic nerve, choroid (>2 mm), sclera, orbit, anterior chamber | |||
| • Tumor in or on the ciliary body • Iris neovascularisation |
|||
| • Tumor necrosis with aseptic orbital cellulitis • Phthisis bulbi |
*, refers to 3 mm in basal dimension or thickness. RB, retinoblastoma.
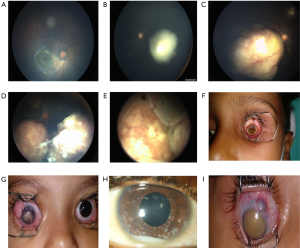
Table 6
| Staging | Characteristics |
|---|---|
| Stage 0 | Unilateral or bilateral RB and no enucleation |
| Stage I | Enucleation with complete histological resection |
| Stage II | Enucleation with microscopic tumor residual (anterior chamber, choroid, optic nerve, sclera) |
| Stage III | Regional extension |
| (I) Overt orbital disease | |
| (II) Preauricular or cervical lymph node extension | |
| Stage IV | Metastatic disease |
| A. Hematogenous metastasis | |
| (I) Single lesion | |
| (II) Multiple lesions | |
| B. CNS extension | |
| (I) Pre-chiasmatic lesion | |
| (II) CNS mass | |
| (III) Leptomeningeal disease |
High-risk features (HRFs)
Clinical HRFs are:
- Extensively necrotic RB is associated with extraocular extension, optic nerve invasion, or choroidal invasion;
- Older age, the long lag period between onset and diagnosis, hyphema, pseudohypopyon, staphyloma, and orbital cellulitis are other important high-risk factors;
- Delay in enucleation and the use of pre-enucleation chemotherapy in group E RB can potentially downstage pathologic evidence of extraocular extension.
Histopathological risk factors
The most important poor prognostic factor for RB is metastasis. Clinical findings may not be useful for predicting the metastasis in RB even though histopathological data may provide a fair estimate of metastasis risk. Patients presenting with glaucoma and/or buphthalmos have a significantly higher risk for histopathologic risk factors, including those resulting in microscopically residual disease after surgery (26). The presence of tumors in the optic nerve posterior to the lamina cribrosa at the site of surgical transection and extrascleral extension of tumor into the orbit is the most important post-surgical prognostic indicator for the development of metastasis (27,28). In a study by Gupta et al., the HRFs were present in 20.4% of children in the developed countries, and 54.2% of the children in the developing countries (29). The increased risk of metastasis seen in the developing countries may be due to a delay in diagnosis and treatment, but the possibility of different biological behavior of tumors may be a contributing factor. Table 7 enlists the histologic HRF.
Table 7
| Site | Type of involvement/risks; characteristics | Risk of metastatic disease |
|---|---|---|
| Choroid | Focal invasion <3 mm | Not increased |
| Massive invasion ≥3 mm | Increased | |
| Optic nerve | Prelaminar | Not increased |
| Lamina cribrosa | Not increased | |
| Postlaminar | Increased | |
| At surgical margin | Increased | |
| Subarachnoid | Increased | |
| Others | Anterior segment invasion | Increased |
| Neovascularization of iris with glaucoma | Increased | |
| Extensively necrotic retinoblastoma | Increased | |
| Buphthalmus | Increased |
Optic nerve invasion
The extent of optic nerve invasion by tumor determines the prognosis of RB. The relation of optic nerve invasion and mortality is mentioned in Table 8 (30). The optic nerve stump measuring <5 mm attached to the enucleated eye have a worse prognosis than those having >5 mm stumps (26). In the histopathology reports, one should include the level of invasion of the optic nerve (prelaminar, laminar, postlaminar, at the cut margin, or/and subarachnoid space of optic nerve).
Table 8
| Extent of optic nerve invasion | Mortality rate (%) |
|---|---|
| Optic nerve is not involved | 10 |
| Superficial invasion | 10 |
| Presence of tumor up to the lamina cribrosa | 29 |
| Invasion of tumor posterior to the lamina cribrosa | 42 |
| The presence of tumor at the transected surgical margin | 80 |
Trilateral RB
Trilateral RB is the presence of unilateral RB or bilateral RB along with a primary intracranial neuroectodermal tumor in the pineal or suprasellar region. They display varying degrees of neuronal differentiation. Most trilateral RBs occur in the pineal region and are histologically pineoblastoma (31). About a fourth of the trilateral RB occurs in the suprasellar or parasellar region. The association of bilateral RB with a pineal tumor and another primary suprasellar tumor is termed quadrilateral RB. A germline mutation in the RB1 gene increases the risk of developing a trilateral RB. The ones with a familial RB have a higher risk of developing a trilateral RB when compares to the de novo germline ones. The median age of patients at diagnosis of trilateral RB is 23–48 months, with an interval of approximately 21 months between diagnosis of bilateral RB and the brain tumor. Trilateral RB has a very poor prognosis (32). Gupta et al. reported (69 cases) the association of pineal cysts with inherited RB and most of these cysts behaved in a benign manner (33).
Metastatic RB
The incidence of metastasis in RB is <5%. The commonest situation in which metastasis is found is after a relapse following enucleation. Most commonly, metastasis occurs to the CNS, bone, and bone marrow. Metastasis can occur by direct invasion of the optic nerve, dispersion through the subarachnoid space of optic nerve, hematogenous route, and lymphatic spread.
Management of RB
The management of RB involves a team consisting of pediatric oncologists, ophthalmologists, radiologists, radiation oncologists, child psychologists, social workers, nurses, and genetic counselors. The most important goal of treatment is to save the patient’s life and to salvage the eye and/or vision. The therapy is decided depending on the status of metastasis, risks for second cancers, systemic involvement, laterality of the disease, size, and location of the tumor(s), and visual prognosis. The treatment options currently available for RB include focal therapy, enucleation, and systemic chemotherapy for possible metastatic disease, and orbital exenteration. These can be used in combination or as an isolated modality.
Focal therapies
The aim of focal therapy is to manage RB with minimal adverse effects on surrounding normal retina and general systemic health.
- Cryotherapy;
- Laser photocoagulation;
- Thermotherapy and chemothermotherapy;
- Plaque brachytherapy;
- External beam radiotherapy (EBRT);
- Transpupillary thermotherapy (TTT).
Cryotherapy
Transscleral cryotherapy involves freezing the tumor under visualization by using indirect ophthalmoscopy. Triple freeze-thaw cryotherapy destroys the tumor cells mechanically by disruption of the cell membranes during the thawing of the intracellular ice crystals. The cryotherapy is repeated every 3–4 weeks. Cryotherapy usually fails if there are overlying vitreous seeds. In unsuccessful cases, plaque radiotherapy is usually employed. Indications of cryotherapy are subretinal seeds and peripheral tumors ≤3.5 mm in diameter and ≤2 mm in thickness. It can also take care of focal vitreous seeds overlying the tumor. Transient serous retinal detachment, retinal tear, and fibrosis are potential complications (34).
Laser photocoagulation
Laser photocoagulation can be used to treat small posterior RB using argon laser, diode laser, or xenon arc photocoagulation. It is selected for tumors ≤4.5 mm in the base and ≤2.5 mm in thickness without vitreous seeds (35). The purpose of treatment is to delimit the tumor and coagulate all blood supply to the tumor. The indirect ophthalmoscope laser photocoagulation system has greatly improved the facility of laser delivery. Two or three sessions at monthly intervals are usually sufficient to control most tumors. Approximately 70% of the tumor control rate can be achieved with laser treatment. Recurrences are often treated with plaque radiotherapy. Small posterior tumors which are 4 mm in diameter and 2 mm in thickness best respond to this modality and it can be used when TTT is not available. Transient serous retinal detachment, visually significant retinal vascular occlusion, retinal traction, retinal hole, and preretinal fibrosis are complications that can be encountered.
Thermotherapy and chemothermotherapy
Thermotherapy uses radiation (ultrasound, microwaves, or infrared) to deliver heat to the eye. The target is to achieve a temperature that is below the coagulative threshold sparing the retinal vessels. Chemothermotherapy is a combination of heat and chemotherapy, and thermoradiotherapy is a combination of heat and radiation. The heat has a synergistic effect with chemotherapy and radiation therapy to treat cancers (36). The therapy selection depends on many factors including tumor size, location, laterality, the status of the opposite eye, presence of subretinal fluid and seeds, presence of vitreous seeds.
Thermotherapy alone can often be used to treat small RBs measuring ≤3 mm without vitreous or subretinal seeds and outside the retinal vascular arcades. With thermotherapy alone, the goal is to heat the tumor to 45 to 60 °C for 10 minutes which would leave a gray-white scar at the site. With chemothermotherapy, the goal is to heat the tumor to 42 to 45 °C for 5 to 20 minutes depending on the tumor size and location. Tumors up to 15 mm in the base can be adequately treated with chemothermotherapy.
The success of this combined therapy is dependent on the careful identification of suitable tumors. Smaller tumors without subretinal fluid or tumor seeds prove the best response. With the use of thermotherapy and chemothermotherapy for RB, tumors ≤3 mm in base respond best with complete control. Tumors ≥6 mm in the base are at increased risk for recurrence of the main tumor and require plaque radiotherapy. Chemothermotherapy is especially suited for small tumors adjacent to the fovea and optic nerve where radiation or laser photocoagulation may induce more profound visual loss.
Plaque brachytherapy
In plaque brachytherapy, a radioactive implant is placed on the sclera over the base of a RB with the intent of irradiating transsclerally. It is used in tumors ≤16 mm in the base and ≤8 mm in thickness. It can be used as either a primary or secondary treatment. In 70% of cases, plaque radiotherapy is used as a secondary treatment to salvage a globe after the failure of prior treatment, usually failed EBRT or chemotherapy (37). There is around 90% tumor control rate with one application of plaque radiotherapy (38). The effective treatment requires an average of 2–4 days of treatment time to deliver the total dose of 4,000 cGy to the tumor apex. The visual outcome depends on size, location, and associated radiation toxicity. The common complication is radiation retinopathy and papillopathy which manifest at approximately 18 months, and are more often seen in children who have been exposed to systemic chemotherapy. Because of the use of focal, shielded radiation fields, plaque radiotherapy is not associated with an increased risk of a second malignancy. Radioisotopes like Iodine-125 and Ruthenium-106 are used for the treatment of various ocular tumors including RB.
Recurrent tumors that are not suitable for treatment by other forms of focal therapy (TTT, cryotherapy, or laser photocoagulation) can be treated with plaque brachytherapy. It has the advantages of direct treatment of the tumor with minimal scarring, deeper penetration, and a single treatment session. The surrounding healthy tissue is not affected and overlying focal vitreous seeds are also treated simultaneously. However, it is not effective in large or multifocal recurrences.
EBRT
EBRT is a method of delivering whole eye irradiation to treat advanced RB particularly when there is diffuse vitreous seeding. EBRT as a lens-sparing technique with an electron beam and a photon beam using a linear accelerator has been employed and it has shown to improve the eye preservation rate. EBRT is also given to bilateral advanced disease with diffuse vitreous seeds as a last globe preserving modality in patients refusing bilateral enucleation after the failure of all other conservative treatment modalities. Newer techniques using stereotactic radiation therapy (SRT), intensity-modulated radiation therapy (IMRT), and proton therapy are available. The standard dose is 20–45 Gy, generally given in fractionated doses over 3–4 weeks.
The rate of ocular salvage depends on the stage of the disease at the time of treatment as well as on the availability of focal therapy for limited recurrences. Recurrence of RB after EBRT is reported within the first 1 to 4 years after treatment. Tumor recurrence is related to the stage of the disease and the size of the largest tumor at the time of treatment.
EBRT is indicated when HRF are present on pathology after enucleation, as a part of multimodal management in orbital RB and case of the tumor and/or vitreous seeds refractory to other treatments. Although it prevents orbital recurrence and achieves excellent long-term tumor control for refractory tumor/vitreous seeds it can cause orbital hypoplasia, dry eye syndrome, secondary malignancies, and cataract (34).
TTT
TTT is a method of applying localized heat to the tissue below the coagulative threshold, thus sparing the retinal vessels from photocoagulation. The goal is to deliver a temperature of 42–60 °C using a diode infrared (810 nm) laser system to induce tumor necrosis. The advantage of TTT is that the scar is smaller than the tumor (36).
Small tumors which are 4 mm in diameter and 2 mm in thickness and subretinal seeds best respond to TTT with a synergistic combination of thermotherapy with chemothermotherapy. It can cause focal paraxial lens opacity, focal iris atrophy, and a large area of retinal scarring, retinal traction, and serous retinal detachment.
Chemotherapy
Intra-arterial chemotherapy (IAC)
IAC is a means for selective delivery of anti-tumor agents to the vicinity of the tumor. A micro-catheter is passed through the femoral artery up to the ophthalmic artery of the eye with the RB. A combination of drugs or a single drug is infused in pulses over 30 min (39). The agents commonly delivered via this route are carboplatin, vincristine, and topotecan. In group B and group C RB, the IAC has a >90% success rate in the salvage of the eye, whereas for group D RB it varies between 45–95% (40).
The duration of treatment may last up to one year with 2–8 procedures required every 4 weeks. The common short term complications are groin hematoma, carotid spasm, ophthalmic, retinal or choroid artery spasm, vitreous hemorrhage, optic neuropathy, headache, and transient neutropenia. A long term risk of choroid toxicity and vision loss are also there. When used as a first-line treatment the globe salvage rate is approximately 75%, and 67% when used as second-line therapy (41).
Intravitreal chemotherapy (IVitC)
Vitreous seeds are aggregates of tumor cells found in the avascular vitreous, which are relatively resistant to the effect of intravenous chemotherapy due to lack of blood supply. These appear due to the disruption of the apical tumor either spontaneously (primary) or treatment-induced necrosis (secondary). IVitC achieves higher drug concentration within the vitreous. It is used as an adjunct along with measures to control the main tumor mass (42). The procedure is performed under anesthesia and a small amount of vitreous is removed. Through a small gauze needle, a small amount of melphalan or topotecan (or combination of both) is injected into space behind the lens into the anterior chamber through the conjunctiva, sclera, or the pars plana. The toxicity of IVitC is limited to technique-dependent localized peripheral retinal toxicity.
With melphalan, vitreous seed regression ranging from 85% to 100% of eyes and globe salvage in 80–100% of eyes has been reported. Intravitreal melphalan is given as a weekly injection until regression. Topotecan can be added to melphalan if the seeds are found to be refractory (43,44).
Subconjunctival chemotherapy
It is administered in conjunction with systemic chemo-reduction in eyes with advanced stages of disease (group D and E) to increase the intraocular concentration of chemotherapeutic agents. A more than 10-fold increase in the concentration of carboplatin can be achieved by this modality. It can be administered up to 3 times a month (16). Side effects include periorbital edema and cellulitis, orbital adipose tissue atrophy, fibrosis of extraocular muscles and tenon’s capsule, and subsequent limitations to ocular motility.
Intravenous chemotherapy (IVC)
IVC is the most widely used treatment modality. The success of IVC in achieving tumor control for early-stage RB is good. It has been used in conjunction with focal therapies for appropriate control of the tumor. The success rates in groups A, B, and C are 100%, 93%, and 90% respectively (41). In group D and E RB, the chemo-reduction with the standard triple-drug regimen has been sub-optimal. Carboplatin-based regimens should be the first choice, but if not available, a regimen including cyclophosphamide and vincristine, with the addition of doxorubicin may be an alternative.
The IVC by itself is rarely sufficient to achieve control and focal therapy is needed for definitive control. It allows the management of RB and lowers the risk of metastasis. Besides, systemic chemotherapy administration has been reported to prevent pineoblastoma and secondary cancers (45). Risks of recurrences are increased in cases where focal therapy is avoided e.g., in the case of macular lesions with the fear of losing vision. The chemotherapy in RB can be employed in three ways:
Standard chemotherapy (chemo-reduction): it is used in the treatment of IORB, both in unilateral and bilateral disease. The aim of chemo-reduction is the shrink the tumor to an extent that focal therapy methods can be applied. The tumor control is approximately 100% for group A eyes and this falls to 40% for group D eyes. Group A IORB however is predominantly managed with focal control measures. Despite encouraging results with chemoreduction for group E eyes, enucleation is preferred for unilateral group E eyes since pre-enucleation chemotherapy can downstage the disease (46).
The main effect occurs in 2–3 cycles when a 35–50% reduction in tumor volumes can be achieved. Due to their good intraocular penetration, the standard chemotherapeutic agents used are vincristine, etoposide, and carboplatin (VEC protocol-Table 9). The chemotherapy is administered every 21–28 days for up to 6 cycles and the reassessment by EUA is done every 2 cycles for the feasibility of local therapy. Cryotherapy, TTT, and plaque RT are the most common focal therapy methods used following chemotherapy.
Table 9
| Drugs | Doses | Schedule | Route |
|---|---|---|---|
| Vincristine | 1.5 mg/m2 (≥3 years) (max. 2 mg); 0.05 mg/kg (<3 years) | Day-1 | IV |
| Carboplatin | 560 mg/m2/day (≥3 years); 18.6 mg/kg/day (<3 years) | Day-1 | IV |
| Etoposide | 150 mg/m2/day (≥3 years); 5 mg/kg/day (<3 years) | Day-1, 2 | IV |
The common problems encountered are tumor recurrences and persistent disease. These issues may require intensified chemotherapy, EBRT, and enucleation.
Adjuvant chemotherapy (chemo-prophylaxis): adjuvant chemotherapy is administered in the setting of the presence of HRFs in the enucleated specimen. Doses used are similar to those employed in chemo-reduction (Table 9). Four to six cycles of adjuvant chemotherapy are recommended. In the presence of an extrascleral extension or extension up to the cut end of the optic nerve additional EBRT is given. In a study by Honavar et al., in 80 patients who had HRFs, who received adjuvant chemotherapy only 4% developed metastasis compared to 24% of those who did not get adjuvant chemotherapy (47).
Neo-adjuvant chemotherapy (NACT) (high dose chemotherapy): EORB has poor outcomes. The current treatment approach is reducing the tumor mass with NACT (thereby avoiding exenteration), followed by enucleation, EBRT to the orbit, and subsequent adjuvant chemotherapy. The doses of the drugs administered are higher (Table 10). In most cases, adequate reduction in tumor volume can be achieved after 3–6 cycles of neoadjuvant chemotherapy after which enucleation is done (48). The efforts to perform enucleation as soon as possible should be made as to the chances of chemoresistance increase with the increase in the number of cycles. After enucleation, chemotherapy is continued for a total of 9–12 cycles. Orbital radiotherapy of 40 Gy dose is administered within 2 months following enucleation. Radhakrishnan et al. applied this treatment to 28 EORB patients and reported a 40.4% survival over 14.75 months and reported that NACT prevented exenteration (49).
Table 10
| Drugs | Doses | Schedule | Route |
|---|---|---|---|
| Vincristine | 0.025 mg/kg (max. 2 mg) | Day-1 | IV |
| Carboplatin | 28 mg/kg/day | Day-1 | IV |
| Etoposide | 12 mg/kg/day | Day-1, 2 | IV |
Alternative regimens of high dose chemotherapy to be used in settings of refractory disease/progressive recurrent disease
This consists of alternative chemotherapy regimens. A commonly used regimen consists of alternating cycles of anti-neoplastic drugs given in Table 11. Cycles A and B are alternated every 3 weeks. Support with granulocyte colony-stimulating factors (G-CSF) should be started in case of delays in chemotherapy with previous cycles or with serious infections.
Table 11
| Drugs | Doses | Schedule | Route |
|---|---|---|---|
| Cycle A | |||
| Vincristine | 1.5 mg/m2/day or 0.05 mg/kg for children <3 y (max. 2 mg) | Day-1 | IV |
| Cyclophosphamide | 65 mg/kg/day | Day-1 | IV |
| Idarubicin OR Doxorubicin | 10 mg/m2/day OR 30 mg/m2 | Day-1 | IV |
| Cycle B | |||
| Carboplatin | 560 mg/m2/day or 18.6 mg/kg for children <3 y | Day-1, 2 | IV |
| Etoposide | 100 mg/m2/day or 3.3 mg/kg for children <3 y | Day-1, 2, 3 | IV |
The combination of vincristine, cyclophosphamide, and idarubicin/doxorubicin can also be used as upfront standard chemotherapy at centers where carboplatin is not available.
In case of non-responsiveness high dose regimens consisting of ifosfamide (1.8 g/m2/day ×5 days) and etoposide (100 mg/m2/day ×5 days) may be given to achieve control. This regimen is also used for initial control in the case of metastatic disease (50).
The combination of topotecan and vincristine has also been found to be effective in a few studies. With the use of GCSF to alleviate neutropenia this treatment was effective as window phase chemotherapy in children with advanced bilateral IORB to allow for subsequent focal therapy to be administered. The majority of the children then went on to receive further chemotherapy and attained a good response. Topotecan was administered at 3 mg/m2/day and the vincristine dose was 0.05 mg/kg for patients age <12 months and 1.5 mg/m2 for patients age ≥12 months at diagnosis (51).
Recurrent RB
Recurrent disease in the form of recurrent tumors and seeding may be more resistant to chemotherapy and radiation. Tumor recurrences when focal therapy has not been given have been reported in 35–40% and about 17% if focal consolidative therapies have been administered. The intraocular recurrent disease has been documented in the retina, vitreous, and subretinal regions. In the presence of a poor visual prognosis, particularly in the presence of a UL RB, enucleation should be considered in the scenario of recurrence of the disease. Chemotherapy and focal therapy may be considered depending on the nature of the recurrence if the vision is salvageable with close follow-up (34).
Intensified chemotherapy and autologous hematopoietic stem cell transplant
This is the only curative modality for metastatic disease. The use of intensified chemotherapy with autologous stem cell transplant (ASCT) has offered some success. The involvement of the CNS has a poorer outcome. However, most of the experience is in stage 4A disease that does not involve the CNS. The use of radiotherapy and intrathecal chemotherapy for CNS lesions has been recommended, although the outcome remains poor (52). With a combination of carboplatin, etoposide, cyclophosphamide, etoposide, and melphalan combined with the support of autologous stem cells a 50–75% long term survival can be achieved (53).
Surgery for RB
Enucleation
Enucleation is done as a part of surgical management for RB, where the major surrounding muscles along with the affected eyeball are removed. The optic nerve end is cut and the surgeon then places an artificial eye to retain the volume of the globe.
Enucleation results in the eye getting sacrificed but achieves a 99% cure for the RB in that eye (54). Enucleation is done in cases of most group E eyes as in them the chances of vision salvage are poor and the risk of metastasis is high. As the enucleated specimen is always submitted for histopathological examination, enucleation offers the advantage of the identification of HRF in the eye which may warrant more aggressive treatment (47).
Unilateral disease with no salvageable vision is best treated by enucleation with the advantage that the patient can be rid of the disease for life, so sometimes group D eyes also end up getting enucleated if the vision is non-salvageable. Enucleation should be done for eyes with EORB which after NACT has shown sufficient reduction in volume that a complete resection can be achieved.
During the procedure, care must be taken not to perforate the eyeball else that will result in tumor seeding the tract. Good cosmetic outcome is achieved by replacement of the volume of the eye with an implant deep in the orbit and provision of a prosthetic eye, which is worn in the conjunctival sac behind the eyelids. A primary orbital implant (silicone, polymethylmethacrylate, porous polyethylene, or hydroxyapatite) placed in the socket provides adequate static and dynamic cosmesis (34).
Exenteration
Exenteration is the removal of the majority of the contents of the orbit and is disfiguring and is indicated for primary/recurrent orbital disease that fails to respond to NACT.
A summary of treatment options for RB depending on the group and stage has been provided in Table 12 and chemotherapy in special circumstances has been provided in Table 13.
Table 12
| RB group | General features | Treatment |
|---|---|---|
| A | Small tumor away from fovea and optic disc | Laser photocoagulation/ |
| Thermotherapy/ | ||
| Cryotherapy/ | ||
| Plaque brachytherapy | ||
| B | Larger tumor | Laser photocoagulation/ |
| Macular | Thermotherapy/ | |
| Juxtapapillary | Cryotherapy/ | |
| Subretinal fluid | Plaque brachytherapy/ | |
| IV/IA chemoreduction | ||
| C | Focal seeds | IAC/ |
| IVitC | ||
| D | Diffuse seeds | IAC/ |
| IVitC/ | ||
| Enucleation | ||
| E | Extensive tumor | Enucleation and adjuvant IV chemotherapy if HRF present ± IAC |
| EORB | Locally advanced disease | NACT and |
| Enucleation and | ||
| EBRT |
IAC, intra-arterial chemotherapy; IVitC, intravitreal chemotherapy; HRF, high risk features; EORB, extraocular retinoblastoma; NACT, neo-adjuvant chemotherapy; EBRT, external beam radiotherapy.
Table 13
| Circumstance | Strategy |
|---|---|
| Recurrent disease after chemotherapy | Can use the initial chemotherapy if initial tumor was chemosensitive or patient is chemonaive followed by local control measures |
| Alternative regimens should be used if at recurrence disease is progressive or extra ocular followed by local control as per stage of the disease | |
| No response to chemotherapy | Alternative chemoreduction regimen can be tried followed by local control as per stage of the disease |
| Metastatic disease | Neo-adjuvant chemotherapy with high dose regimen |
| Local control (enucleation and radiotherapy if needed) | |
| Conditioning chemotherapy with carboplatin, etoposide and cyclophosphamide followed by stem cell rescue |
Gene therapy
Suicide gene therapy is based on the introduction of a viral or bacterial gene into tumor cells, thus allowing the conversion of a nontoxic compound into a lethal drug to kill the tumor cells. Injections of an adenovirus vector containing HSV-tk (Herpes Simplex virus type-1 thymidine kinase), given intravitreally, followed by treatment with ganciclovir was shown to be safe and effective against vitreous seeds. Although this is still experimental it is unlikely that gene therapy could be used as first-line treatment for RB, but its use may be better suited as an adjunct to standard therapy for the treatment of refractory vitreous seeds (56).
Prognosis
The prognosis of RB is excellent with a cure rate of 95% in developed countries. The preservation of vision depends on many factors including the initial tumor volume, the anatomical relationship of the tumors with the macula and the optic disc, and the adverse effects of the treatments. Patients treated for hereditary RB are at an increased risk of developing non-ocular malignancies due to a mutation in the second RB1 allele in different tissues. The development of tumors in the contralateral eye after 3 years is very rare. If the tumors are small and distant from the fovea, the prognosis for vision is good after successful treatment.
Follow up plans
Patients with RB who undergo any form of eye salvaging treatment need regular follow-up EUA to monitor for recurrence. The tumor regression and the appearance, size, location, and the number of tumors should be documented and followed very closely during each examination. After treatment, when a tumor regresses, it can appear as a white, calcific mass. Recurrence of RB from treated lesions is very common and can occur years after treatment. In cases where chemoreduction therapy has been administered, the examination should be done every 3–4 weeks with each cycle of chemotherapy. The EUA should be done every 4–8 weeks for patients who receive focal therapy until complete tumor regression. Following tumor regression, subsequent EUA should be every 3 months for the first year, every 6 months for 3 years or until the child reaches 5 years of age, and yearly ophthalmoscopic examination thereafter.
Late effects
The common late effects in RB survivor patients include growth retardation (one-third of patients), diminished vision (one-sixth of patients), orbital hypoplasia and contracted socket (14.1%), hearing impaired (2.7%), global intelligence delay (one-sixth) and rarely second neoplasms (57). EBRT may induce a second malignancy in the field of irradiation. The 30-year cumulative incidence for second cancers in bilateral RB has been reported to be 35% for patients who receive radiation therapy (58).
EBRT, when administered before the first year of life, and chemotherapy may also increase the risk of development of second neoplasms. The most frequent tumors encountered are osteosarcoma of the skull and long bones, soft tissue sarcomas, cutaneous melanomas, brain tumors, lung, and breast cancer.
Summary
The management of RB needs holistic care. It requires the selection of the best treatment option for the patient and careful monitoring for recurrences. Enucleation should be performed in advanced RB with no visual prognosis. Primary focal therapy with laser photocoagulation, TTT, and cryotherapy for peripheral tumors are used for IORB. Systemic chemotherapy continues to be the standard treatment for IORB groups B to D and bilateral RB. Appropriate use of chemotherapy can salvage group D eyes. Group E, IORB are ideally enucleated upfront, and chemotherapy is only administered if HRF is present in the histopathology. EORB requires systemic chemotherapy, enucleation, and EBRT. IAC is a very promising treatment with high success for advanced RB. IVitC should be performed with safety-enhanced techniques and has a high success rate for vitreous seeds. Radiotherapy should be employed only in selected cases. Specific precautions during the surgery, use of a primary implant, and post-enucleation evaluation of histopathologic HRFs and adjuvant therapy achieve optimal life salvage. RB has a very good survival rate.
Acknowledgments
We acknowledge Dr. Neiwete Lomi, Assistant Professor of Ophthalmology, Dr. Rajendra Prasad Centre for Ophthalmic Sciences, AIIMS, New Delhi for providing the images for this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Pediatric Medicine. The article was sent for external peer review organized by the editorial office.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/pm-20-79
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm-20-79). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Comings DE. A general theory of carcinogenesis. Proc Natl Acad Sci USA 1973;70:3324-8. [Crossref] [PubMed]
- Friend SH, Bernards R, Rogelj S, et al. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 1986;323:643-6. [Crossref] [PubMed]
- Knudson AG Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 1971;68:820-3. [Crossref] [PubMed]
- Kivelä T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol 2009;93:1129-31. [Crossref] [PubMed]
- Shields JA, Shields CL. Intraocular tumors-a Text and Atlas. Philadelphia: WB Saunders Company, 1992.
- Dimaras H, Kimani K, Dimba EA, et al. Retinoblastoma. Lancet. 2012;379:1436-46. [Crossref] [PubMed]
- Albert DM. Historic review of retinoblastoma. Ophthalmology 1987;94:654-62. [Crossref] [PubMed]
- Gupta AK, Meena JP, Seth R. Reasons for missed chemotherapy appointments in Retinoblastoma patients undergoing chemotherapy: A Report from a Tertiary Care Hospital from India. Cancer Rep (Hoboken) 2020. [Epub ahead of print].
- Lohmann DR. RB1 gene mutations in RB. Hum. Mutat 1999;14:283-8. [Crossref] [PubMed]
- Thériault BL, Dimaras H, Gallie BL, et al. The genomic landscape of RB: a review. Clin. Experiment. Ophthalmol 2014;42:33-52. [Crossref] [PubMed]
- Gallie B, Erraguntla V, Heon E, et al. RB. In: Taylor D, Hoyt C. editors. Pediatric Ophthalmology and Strabismus. 3rd ed. London: Elsevier, 2004:486-504.
- Athavale V, Khetan V. Knudson to embryo selection: A story of the genetics of RB. Taiwan J Ophthalmol 2018;8:196-204. [Crossref] [PubMed]
- Soliman SE, Racher H, Zhang C, et al. Genetics and molecular diagnostics in RB - An update. Asia Pac J Ophthalmol (Phila) 2017;6:197207.
- Valenzuela A, Chan HSL, Heon E, et al. A language for RB: guidelines through standard operating procedures. In: Reynolds J, ed. Pediatric Retina. New York: Marcel Dekker Inc;2010:205-34.
- Impact Genetics. RB Risks and Surveillance Plans. 2016. Available online: http://impactgenetics.com/wp-content/uploads/2012/10/RB-risks-and-surveillance-plans.pdf
- Mendelsohn ME, Abramson DH, Madden T, et al. Intraocular concentrations of chemotherapeutic agents after systemic or local administration. Arch Ophthalmol 1998;116:1209-12. [Crossref] [PubMed]
- Vemuganti G, Honavar SG, John R. Clinicopathological profile of retinoblastoma in Asian Indians. Invest Ophthalmol Visual Sci 2000;41:790.
- Ata-ur-Rasheed M, Vemuganti G, Honavar S, et al. Mutational analysis of the RB1gene in Indian patients with retinoblastoma. Ophthalmic Genet 2002;23:121-8. [Crossref] [PubMed]
- Brisse HJ, Lumbroso L, Freneaux PC, et al. Sonographic, CT, and MR imaging findings in diffuse infiltrative retinoblastoma: report of two cases with histologic comparison. AJNR Am J Neuroradiol 2001;22:499-504. [PubMed]
- Rootman DB, Gonzalez E, Mallipatna A, et al. Hand-held high-resolution spectral-domain optical coherence tomography in retinoblastoma: clinical and morphologic considerations. Br J Ophthalmol 2013;97:59-65. [Crossref] [PubMed]
- Shields J, Shields C. Retinoblastoma: diagnostic approaches. In: Shields J, Shields C. editors. Atlas of intraocular tumors, 3ed. Philadelphia, PA: Lippincott, Wolters Kluwer, 2016.
- Reese AB, Ellsworth RM. Management of retinoblastoma. Ann N Y Acad Sci 1964;114:958-62. [Crossref]
- Shields CL, Mashayekhi A, Demirci H, et al. Practical approach to the management of retinoblastoma. Arch Ophthalmol 2004;122:729-35. [Crossref] [PubMed]
- Linn Murphree A. Intraocular retinoblastoma: The case for a new group classification. Ophthalmol Clin North Am 2005;18:41-53. [Crossref] [PubMed]
- Shields CL, Shields JA. Basic understanding of current classification and management of retinoblastoma. Curr Opin Ophthalmol 2006;17:228-234. [Crossref] [PubMed]
- Chantada GL, Gonzalez A, Fandino A, et al. Some clinical findings at presentation can predict high-risk pathology features in unilateral retinoblastoma. J Pediatr Hematol Oncol 2009;31:325-9. [Crossref] [PubMed]
- Shields CL, Shields JA, Baez K, et al. Optic nerve invasion of retinoblastoma: metastatic potential and clinical risk factors. Cancer 1994;73:692-8. [Crossref] [PubMed]
- Mohney BG, Robertson DM. Ancillary testing for metastasis in patients with newly diagnosed retinoblastoma. Am J Ophthalmol 1994;118:707-11. [Crossref] [PubMed]
- Gupta R, Vemuganti GK, Reddy VA, et al. Histopathologic risk factors in retinoblastoma in India. Arch Pathol Lab Med 2009;133:1210-4. [PubMed]
- Chantada GL, Fandino A, Mato G, et al. Phase II window of idarubicin in children with extraocular retinoblastoma. J Clin Oncol 1999;17:1847-50. [Crossref] [PubMed]
- Paulino AC, Trilateral RB. Is the location of the intracranial tumor important? Cancer 1999;86:135-41. [Crossref] [PubMed]
- De Potter P, Shields CL, Shields JA. Clinical variations of trilateral RB: A report of 13 cases. J Pediatr Ophthalmol Strabismus 1994;31:26-31. [PubMed]
- Gupta AK, Jones M, Prelog K, et al. Pineal cysts-A benign association with familial retinoblastoma. Pediatr Hematol Oncol 2016;33:408-14. [Crossref] [PubMed]
- Rao R, Hanovar SG. Retinoblastoma. Indian J Pediatr 2017;84:937-44. [Crossref] [PubMed]
- Shields JA. The expanding role of laser photocoagulation for intraocular tumors. 1993 H. Christian Zweng Memorial Lecture. Retina 1994;14:310-22. [Crossref] [PubMed]
- Shields CL, Santos MC, Diniz W, et al. Thermotherapy for retinoblastoma. Arch Ophthalmol 1999;117:885-93. [Crossref] [PubMed]
- Shields CL, Shields JA, De Potter P, et al. Plaque radiotherapy in the management of retinoblastoma: use as a primary and secondary treatment Ophthalmology 1993;100:216-24. [comments]. [Crossref] [PubMed]
- Shields CL, Shields JA, Minelli S, et al. Regression of retinoblastoma after plaque radiotherapy. Am J Ophthalmol 1993;115:181-7. [Crossref] [PubMed]
- Suzuki S, Yamane T, Mohri M, et al. Selective ophthalmic arterial injection therapy for intraocular RB: the long-term prognosis. Ophthalmology 2011;118:2081-7. [Crossref] [PubMed]
- Shields CL, Manjandavida FP, Lally SE, et al. Intra-arterial chemotherapy for RB in 70 eyes: outcomes based on the international classification of RB. Ophthalmology 2014;121:1453-60. [Crossref] [PubMed]
- Shields CL, Mashayekhi A, Au AK, et al. The international classification of RB predicts chemo reduction success. Ophthalmology 2006;113:2276-80. [Crossref] [PubMed]
- Munier FL, Gaillard MC, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in RB revisited: from prohibition to conditional indications. Br. J. Ophthalmol 2012;96:1078-83. [Crossref] [PubMed]
- Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from RB. Arch Ophthalmol 2012;130:1268-71. [Crossref] [PubMed]
- Francis JH, Abramson DH, Gaillard MC, et al. The classification of vitreous seeds in RB and response to intravitreal melphalan. Ophthalmology 2015;122:1173-9. [Crossref] [PubMed]
- Ramasubramanian A, Kytasty C, Meadows AT, et al. Incidence of pineal gland cyst and pineoblastoma in children with RB during the chemo reduction era. Am J Ophthalmol 2013;156:825-9. [Crossref] [PubMed]
- Zhao J, Dimaras H, Massey C, et al. Pre-enucleation chemotherapy for eyes severely affected by retinoblastoma masks risk of tumor extension and increases death from metastasis. J Clin Oncol 2011;29:845-51. [Crossref] [PubMed]
- Honavar SG, Singh AD, Shields CL, et al. Postenucleation adjuvant therapy in high-risk RB. Arch Ophthalmol 2002;120:923-31. [Crossref] [PubMed]
- Honavar SG, Singh AD. Management of advanced RB. Ophthalmol Clin North Am 2005;18:65-73. [Crossref] [PubMed]
- Radhakrishnan V, Kashyap S, Pushker N, et al. Outcome, pathologic findings, and compliance in orbital RB (International RB Staging System stage III) treated with neoadjuvant chemotherapy: a prospective study. Ophthalmology 2013;119:1470-7. [Crossref] [PubMed]
- Chantada G, Luna-Fineman S, Sitorus RS, et al. SIOP-PODC recommendations for graduated-intensity treatment of retinoblastoma in developing countries. Pediatr Blood Cancer 2013;60:719-27. [Crossref] [PubMed]
- Qaddoumi I, Billups CA, Tagen M, et al. Topotecan and vincristine combination is effective against advanced bilateral intraocular retinoblastoma and has manageable toxicity. Cancer 2012;118:5663-70. [Crossref] [PubMed]
- Dunkel IJ, Khakoo Y, Kernan NA, et al. Intensive multimodality therapy for patients with stage 4a metastatic RB. Pediatr Blood Cancer 2010;55:55-9. [PubMed]
- Matsubara H, Makimoto A, Higa T, et al. A multidisciplinary treatment strategy that includes high-dose chemotherapy for metastatic RB without CNS involvement. Bone Marrow Transplant 2005;35:763-6. [Crossref] [PubMed]
- Shields CL, Fulco EM, Arias JD, et al. RB frontiers with intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Eye 2013;27:253-64. [Crossref] [PubMed]
- Mendoza PR, Grossniklaus HE. Therapeutic options for retinoblastoma. Cancer Control 2016;23:99-109. [Crossref] [PubMed]
- Chévez-Barrios P, Chintagumpala M, Mieler W, et al. Response of RB with vitreous tumor seeding to adenovirus-mediated delivery of thymidine kinase followed by ganciclovir. J Clin Oncol 2005;23:7927-35. [Crossref] [PubMed]
- Seth R, Singh A, Guru V, et al. Long-term follow-up of retinoblastoma survivors: Experience from India. South Asian J Cancer 2017;6:176-9. [Crossref] [PubMed]
- Dudgeon J. Retinoblastoma: trends in conservative management. Br J Ophthalmol 1995;79:104. [Crossref] [PubMed]
Cite this article as: Gupta AK, Meena JP. A narrative review of retinoblastoma and recent advances in its management. Pediatr Med 2020;3:20.