
Undernutrition in critically ill children
Introduction
Globally, in 2018, 49 million (7.3%) children under 5 years presented acute malnutrition (wasting), and 149 million (21.9%) children under 5 years suffered from stunting (1). Malnutrition remains a leading cause of childhood mortality. According to the Global Burden of Disease Study 2013, the number of deaths for protein-energy malnutrition, age-standardized, was 9.8/1,000,000 in children and adolescents aged 0 to 19 years in the 50 most populous countries. Considering the level of development, malnutrition-related death was 11/100,000 in developing and 0.1/100,000 in developed countries (2).
Malnutrition is common, but underreported, among hospitalized children. Poor nutritional status (NS) at admission or worsening of NS during hospitalization is recognized to adversely affect clinical outcomes and increase healthcare costs. Thus, there is a need for better identification, documentation, and coding of pediatric malnutrition (3-5).
Mehta and colleagues suggest a new paradigm for defining pediatric malnutrition that includes the chronicity, etiology, mechanisms, severity, and the impact on the outcomes. In addition, obesity and excess energy intake can be also classified as malnutrition (6). For the purpose of this study, we will consider malnutrition only as pediatric undernutrition.
Despite efforts to properly define and report pediatric malnutrition (6,7), there is still no consensus on the best definition. The disparity of the reported prevalence of undernutrition in hospitalized children, ranging from 2.4% to 39.7%, depends on the population studied, clinical settings, parameters, and classification methods (3).
In critically ill children, the reported prevalence of undernutrition at pediatric intensive care unit (PICU) admission range from 8.1% (8) to 71.7% (9), but the differences in nutritional indices, presence of chronic illness, age and severity of critical illness should be considered when evaluating these numbers. Critically ill children are also at risk of nutritional deterioration due to the illness itself and inadequacy of nutrient delivery (10,11).
Early identification of pediatric undernutrition and the recognition of NS deterioration can lead to prompt and adequate nutritional interventions, which may improve clinical outcomes. This review aimed to report and discuss the undernutrition identification at PICU admission and during PICU stay, and also, to discuss the implications of undernutrition on outcomes in critically ill children.
Undernutrition in pediatric critical illness: definition and evaluation
Metabolic response to stress
In critical illness, the metabolic response to acute conditions, like trauma, infection, or surgery, leads to neuroendocrine, metabolic, and immunologic modifications. The main characteristics are the protein turnover amplified with an increase in hepatic protein synthesis and negative protein balance, insulin resistance, and consequently hyperglycemia and increased lipolysis (12,13). Pediatric critical illness can be characterized in three phases: acute, stable, and recovery phase (12,14). The acute phase, which can last for hours to days after an event, is represented by severe catabolism (12,15). It is considered an adaptive response to protein breakdown to provide free amino acids and energy supply to vital tissues triggering an intense catabolic state (14,16,17).
Therefore, due to the negative protein balance, protein loss is frequently observed, which can result in loss of lean body mass (17). Multiple intracellular signaling pathways are responsible for the expression of atrogenes, which triggers proteolysis by the ubiquitin-proteasome complex (UPS) and autophagy. The most predominant proteolytic pathway that leads to muscle atrophy is forkhead box protein O (FOXO) and UPS. It provides negative feedback to the phosphoinositol-3 kinase/protein kinase B (PI3K/AKT) pathway, leading to inhibition of protein synthesis and, together with the accelerated proteolysis, leads to muscular atrophy (18,19). In face of infections and severe inflammation, levels of inflammatory cytokines increase and may amplify the process enhancing the protein breakdown (14). Although studies in pediatrics are scarce, it has been shown in a study conducted with critically ill adults that muscle wasting occurred early and fast during the first week of critical illness (20) (Figure 1).
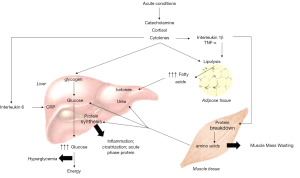
The following phase is characterized by stabilization, but some features of the metabolic response remain unsolved. Finally, in the recovery phase, when there is no need of vital organ support, it is observed the resolution of the stress response and the anabolism state with protein synthesis (12). Therefore, patients can progress to death, recovery, or as described more recently, to chronic critical illness (CCI). Patients that develop this new condition may show multiple phenotypes, including persistent inflammation and immunosuppression. The sustained activation of inflammation may lead to prolonged catabolism with loss of muscle mass (21). The loss of lean body mass that leads to deterioration in muscle function is associated with the protein catabolism, immobilization, and nutritional deficits (22).
NS assessment
The ideal nutritional approach in PICU should include NS assessment of all critically ill children within 48 hours of admission, to identify undernutrition before hospitalization or during PICU stay (11,23).
The NS assessment can be performed by different indicators, such as anthropometric measurements, physical examination, assessment of serum proteins, and/or inflammatory markers (11,24). Although there is no consensus about the best method to evaluate the NS of critically ill children (25), it should combine static nutritional indices and dynamic nutritional assessment before and during PICU stay, to integrate malnutrition etiology and consequences in its interpretation (6,26).
Nutritional risk screening
Nutritional screening tools (NSTs) have been developed to assist in the early detection of children at risk of malnutrition during hospitalization (6,27). Children identified with a high nutritional risk by NSTs are more likely to benefit from an early nutrition intervention and need close monitoring of NS (11,27).
Previous studies applied NSTs in critically ill children, such as the Screening Tool for Risk of Impaired Nutritional Status and Growth tool (STRONGkids) (28) and the Pediatric Yorkhill Malnutrition Score (PYMS) (29,30), and found divergent results regarding the ability of these NSTs to detect malnutrition risk in these population (31-33). Nutritional risk assessed by STRONGkids presented an area under the curve (AUC) of 0.822, 92.1% sensitivity and 61.1% specificity when compared with WAz (32), and was associated with NS assessed by WAz, HAz and BMIz (31,33). In addition, PYMS presented an AUC of 0.759, 76.2% sensitivity and 63.9% specificity when compared with WAz. However, no significance was found in the effectiveness of both NSTs in screening for malnutrition in these population (32). Furthermore, it should be noted that these NSTs were developed for general hospitalized children and excluded PICU patients in their development. To date, no validated NST for critically ill children has been identified or developed, and none of the NSTs developed for hospitalized children are validated for PICU patients (11,25).
Anthropometric parameters
According to the guidelines, it is recommended that weight and height/length (11,34), mid-upper arm circumference (MUAC), and head circumference, in young children <36 months (34), should be measured. The evaluation should include the calculation of the z-scores for body mass index (BMI)-for-age (BMIz) or, weight-for-length (WLz) in children <2 years, or weight-for-age (WAz), if accurate length/height is not available (11,34).
To diagnose undernutrition by anthropometric measurements, reference curves must be used, considering the cut-off points for z-scores established by the World Health Organization (WHO) and/or the Centers for Disease Control and Prevention (CDC) (6). The degrees of undernutrition based on z-scores include “mild or at risk of undernutrition” when z-scores < –1; “moderate undernutrition” when z-scores between –2 and –3; and, “severe undernutrition” when z-scores < –3. The chronicity of undernutrition can be classified as “acute undernutrition” when the duration is lower than 3 months, usually evaluated by WAz, weight-for-height (WHz), or BMIz; or “chronic undernutrition”, lasting for at least 3 months and evaluated by HAz (6,7).
There are many challenges in obtaining anthropometric measurements in critically ill children. In an international survey to characterize the barriers to anthropometric measurement, 84% of the health care providers agreed that anthropometric measurements were important, but only 3% indicated that such measurements were always obtained upon PICU admission. The presence of medical devices, need for extracorporeal life support, and unstable hemodynamic status were the most reported perceived patient-specific barriers (35). Besides these barriers, it is important to highlight that weight measurements should be interpreted in the context of fluid shifts, such as the presence of fluid overload and edema (36).
Growth status
Faltering growth (also called failure to thrive) is considered a dynamic approach that allows to assess growth status and detect impaired growth before reaching undernutrition, according to WHO cut-off points. It is used to describe when children are not growing or gaining weight as expected, and it is defined as a non-voluntary deceleration on WAz, HAz, or BMIz decline greater than 1 z-score (6,26,37).
A history of underlying chronic disease has been shown to correlate with the risk of being malnourished and/or presenting with faltering growth at PICU admission. However, faltering growth assessment is rarely performed, due to the necessity of the children’s previous growth data (26,38).
Laboratory parameters
In addition to anthropometry, laboratory markers can be used as complementary measures in the NS of critically ill children. The role played by serum biomarkers in diagnosing or monitoring undernutrition is still controversial, due to their relatively low specificity and by the fact that they reflect the presence of inflammatory, hypermetabolic, and/or hypercatabolic conditions (6,39,40).
The most commonly used biomarkers in clinical practice include C-reactive protein (CRP), serum albumin, prealbumin, and retinol-binding protein (25,41). While these markers were associated with clinical outcomes such as mortality, length of stay (LOS), and mechanical ventilation independently from the inflammatory response (42-45), studies showing their predictive value on NS are lacking (41).
Although there is no recommendation for the best laboratory parameters, it is clear that the complex role of inflammation and disease etiology must be accounted in the assessment of pediatric undernutrition (11), and novel biomarkers to track NS during critical illness and in response to interventions should be identified (34).
Micronutrient status
Micronutrients such as zinc (Zn), selenium (Se), manganese (Mn), and copper (Cu) play an important role in biological functions including antioxidant defense, immune function, DNA repair, glucose control, and healing (46). However, critical illness and inflammation affect serum/plasma micronutrient status, limiting its assessment in intensive care (46,47). In infants and children, due to the immature organ function, specific age-dependent metabolic demands, and susceptibility to environmental influences, it is more complicated to determine their micronutrient status (46).
Recently, a scoping review of micronutrient status during pediatric critical illness summarized some important gaps in knowledge that must be considered for future studies. To date, there are not enough data to establish micronutrient requirements in pediatric critical illness (47,48). Although there is evidence of micronutrient role in the immune and metabolic responses, the causality of the association between micronutrient levels and clinical outcomes remains unclear, as well as the impact of micronutrient supplementation during PICU admission on those outcomes (46,47).
NS deterioration
NS deterioration is defined as a weight loss or a decline of NS indicators during PICU stay (6,49). Variables such as the severity of illness and PICU LOS may account for NS deterioration, and they may be related to inflammation, oxidative stress (48) and underfeeding in critically ill children. Also, acquired undernutrition during PICU stay and muscle loss may lead to ventilation weaning failure and PICU-acquired weakness (37).
Few studies have evaluated the NS deterioration during PICU stay and there is a lack of standardization of the cut-off points. A prospective study with 579 critically ill children has defined NS deterioration as a weight loss >5% or a decline in BMIz >0.5 SD (37), whereas a case-control study with 80 critically ill children after surgery for congenital heart disease has defined NS deterioration as a decline in WAz >0.67 z-score (50). Other studies with critically ill children evaluated NS deterioration based on the reduction of continuous values of different NS indicators, such as MUAC (cm) (51), triceps skinfold thickness (TSF) (mm) (51), arm muscle area (cm2) (51), WAz (38,51-53), HAz (38), BMIz (51), MUAC for-age z-score (MUACz) (38,51), TSF-for-age (38), biceps skinfold thickness-for-age z-score (38), and calf circumference-for-age z-score (38). Considering that different nutritional indicators and cut-off points are evaluated, it is difficult to compare results between studies.
Muscle mass wasting
In critical illness, negative protein balance with consequent muscle protein loss is higher in comparison to other diseases, and it is even more intense in the most severely ill patients (54). Also, muscle atrophy in mechanical ventilated critically ill children is common and rapid. It can occur within 5–7 days and, age and traumatic brain injury are risk factors associated with greater muscle loss (55).
The proper definition of muscle mass loss and the use of validated and reproducible tools to assess muscle mass in PICU allows the early identification of muscle wasting in the clinical setting (22,56). However, a comprehensive evaluation of muscle loss in critically ill children can be challenging in clinical practice (55,57).
The current methods available to estimate body composition and muscle mass may show some limitations in PICU. Skinfold thickness, MUAC, and bioelectrical impedance analysis (BIA) may not be reliable for muscle changes, since they are affected by hydration states (57). Also, computerized tomography (CT) or magnetic resonance imaging (MRI) are impractical to perform at bedside (22,57,58). In this context, ultrasound, as a noninvasive technique and available at the bedside, may be a promising method to measure skeletal muscle (22,56). Although there are not sufficient studies investigating ultrasound as a surrogate for muscle mass in critically ill children, the quadriceps femoris (QF) muscle is one of the most commonly measured. In a longitudinal study conducted with critically ill children, QF muscle assessed by ultrasound showed an early and clinically important decrease. However, ultrasound may be compromised by the hydration status (56,57).
For muscle wasting detection, assessing skeletal muscle turnover with more complex methods are being studied in critically ill children. In a study with 19 critically ill children after thoracic surgery, a 15N glycine urinary end-product enrichment technique was used to investigate whole-body protein turnover. The method was considered feasible and noninvasive. It was observed an increased protein breakdown resulting in increased protein turnover and net negative protein balance (59).
Therefore, early identification of muscle wasting allows the provision of adequate nutrition therapy and other potential interventions to minimize muscle wasting, and the poor outcomes associated. It is relevant to consider methods for continued measurement of lean mass at the bedside, such as ultrasound or BIA (17,57).
Prevalence of undernutrition in pediatric critical illness
Prevalence of undernutrition at PICU admission
Table 1 describes the definition and prevalence of undernutrition in critically ill children at PICU admission. NS at PICU admission is mainly evaluated by anthropometry. Most of the studies defined undernutrition based on WAz, WHz, or BMIz (z-scores < –2), which evaluate acute undernutrition. The prevalence of acute undernutrition ranged from 7.2% to 64.9% as described in Table 1 and Mehta et al.'s study (104). Some studies also assessed the presence of chronic undernutrition, evaluated by HAz, ranging from 16% to 42.8% (38,44,51,60,70,76,79,89,90,92). Moreover, few studies considered the presence of chronic diseases to evaluate undernutrition (87,88,92). Several studies reported different approaches, z-scores cut-offs, percentiles or weight and height ratios based on Waterlow’s recommendations (9,97-103). The dynamic approach was observed only in three studies, conducted in French PICUs, that reported the prevalence of faltering growth, based on WAz, of 13.7% (26) and 4.8% (67) and 10.2% based on BMIz (37).
Table 1
| Definition | Country/region | Population | Undernutrition (%) | Reference |
|---|---|---|---|---|
| < -2 z-scores | ||||
| WAz, HAz, BMIz, WHz, MUACz | China, Single-center | Pediatric oncology, 58 months [IQR 1–205], severe critical illness: 26.25%; (N=160) | WAz: 11.3%, HAz: 16.3%, BMIz: 21.3%, WHz: 14.4%, MUACz: 34.4% | (60) |
| WHz | Korea, Single-center | MV children, 17.38 months [IQR 0.5–205], PRISM3 17.4 (SD 7.37); (N=70) | 45.7% | (61) |
| <2 years: WHz; >2 years: BMIz | Brazil, Single-center | Children with respiratory insufficiency, 6 months [IQR 2–13], PIM2 ≤4%: 75% (N=71) | 26% | (62) |
| WHz | Singapore, Single-center | Children with severe bronchiolitis, 4.9 months [IQR 2–10.4], 62.2% with multiorgan dysfunction; (N=74) | 20.3% | (63) |
| BMIz | 3 Countries, 31 PICUs | Children with inotropic support and/or MV and developed hyperglycemia, <2 years: 30.4%; (N=608) | 7.2% | (64) |
| BMIz | Brazil, Single-center | Children, 23.1 months [IQR 3.9–89.1], PIM2 4.2 [IQR 1.3–15.7]; (N=199) | 18% | (65) |
| BMIz | France, Single-center | Children, 13.6 months [IQR 1.9–96.1], PIM2 4.5 [1.5–8.5]; (N=579) | 15% | (37) |
| WAz | United States, 12 PICUs | Children, 6.61 years [IQR 1.64–12.92), PIM2 1.34% [IQR 0.79–4.41] (N=2069) | 21.4% | (66) |
| BMIz | France, 27 PICUs | Children, 2.9 years [IQR 0.5–10.6], PIM2 2.1 [IQR 0.8–8.7]; (N=432) | 18.5% | (67) |
| WHz | India, Single center | Children with acute gastroenteritis with severe dehydration, 9.5 months [IQR 5–18], sepsis 61%; (N=62) | 64.9% | (68) |
| WAz | United Kingdom and Netherlands, 2 PICUs | Children aged 0– 12 months, with a PICU stay ≥7 days, 2.6 months [0.3–6.0] (PICU1) and 2.6 months [0.4–3.6] (PICU2); (N=53) | 26.41%; (PICU1): 40%; (PICU2): 14% | (69) |
| WAz, HAz, WHz | United Kingdom, Single-center | MV children, 2 years [IQR 0.6–4.9], PIM2 score 3.2 [IQR 0.9–6.2]; (N=87) | WAz: 15%, HAz: 17%, WHz: 8% | (70) |
| BMIz | Brazil, Single-center | Children, 4.8 years, PIM 2.7; (N=247) | 15% | (71) |
| WAz | Turkey, Single-center | Children, 16.17 months (range 1.43–92.1), PRISM3 10 (range 0–30) (N=81) | 28.3% | (40) |
| BMIz | France, Single-center | Children, 18.8 months [IQR 2.7–103.8], PIM2 2 [IQR 1–6]; (N=683) | 13% | (26) |
| <2 years: WHz; >2 years: BMIz | United States, Single-center | MV children, 9.5 months, PRISM3 ≥10: 26.4%; (N=106) | 7.5% | (72) |
| BMIz | 26 countries, 129 PICUs | Children with severe sepsis, <12 months: 37%; (N=417) | 18% | (73) |
| BMIz | 15 countries, 57 PICUs | MV surgical children, 2 years [IQR 0.5–8]; (N=519) | 19% | (74) |
| WAz | Taiwan, Single-center | Children, 4.4 years (range 0.1–18); (N=282) | 8.2% | (75) |
| WAz, HAz, BMIz | Brazil, Single-center | MV children, 21.1 months [IQR 4.4–82.2], PIM2 8.0 [IQR 1.6–21.1]; (N=72) | WAz: 33.9%; HAz: 41.2% BMIz: 22.1%; | (76) |
| WAz | Rwanda, Single-center | Children, newborn – 15 years, <1 year: 60%; (N=210) | 59%; Severe: 30% | (77) |
| BMIz | 16 countries, 90 PICUs | MV children, 4.5 years (SD 5.1), severe severity of illness: 29%; (N=1622) | 18% | (78) |
| <2 years: WAz, HAz, WHz; >2 years: BMIz | Brazil, Single-center | Children, 34 months [9–85], PIM2 3.12 [1.17–8.57]; (N=271) | 42.4% | (44) |
| WAz, HAz, MUACz | Brazil, Single-center | Children with sepsis, 24 months; (N–14) | WAz: 33.3%; HAz: 42.8%; MUACz: 66.6% | (79) |
| BMIz | India, Single-center | Children, PIM2>15: 23%; (N=332) | 57.2% | (80) |
| WAz | Netherlands, Single-center | Children receiving enteral nutrition (newborns 138, infants 99 and children 88), PRISM 8 [IQR 0–31]; (N=325) | 19% | (81) |
| WAz | 15 countries, 59 PICUs | MV children, 1.7 years [IQR 0.4–7.0], severe severity of illness: 22%; (N=1245) | 25% | (82) |
| WAz | United Kingdom, Single center Two cohorts (2009, 2010) | Children fed exclusively with EN, 0.3 to 49 years, PIM2 0.5–2.3; (N=130) | CHD surgical: 31%; Medical: 15%; General surgical: 18% | (83) |
| WAz | United States and Canada, 12 PICUs | Children, 2.4 years [IQR 0.5–9.8]; (N=5105) | 28.16% | (84) |
| Non reported | Brazil, Single-center | MV children, 28 months (range 1–201) (N=96) | 50% | (85) |
| WAz | Malaysia, Single-center | Children on enteral nutritional support, 10.2 months [IQR 5.1–50.5]; (N=53) | 43.2 | (86) |
| <2 years: WAz; >2 years: BMIz; chronic diseases: HAz | Brazil, Single center | Critically, 2.3 years [0.6–13.5], PIM2 2.9 [1.2–6.5]; (N=281) | 47.1% | (87) |
| WAz, BMIz; chronic diseases: HAz | Brazil, Single center | Children using EN or PN, 11.2 months [2.6–58.3], PIM2 3.5 [1.2–7.9]; (N=207) | 54.1% | (88) |
| acute: WAz; chronic: HAz | United States, Single-center | Children, 1.4 [IQR 0.3–9.4) years, no-AKI: 61%, AKI†: 12%, PRISM3 no-AKI: 3 [IQR 1–7] and AKI†: 7 [IQR 5–10.3]; (N=167) | acute: 20% (AKI: 33%); chronic: 17.6% (AKI: 23.8%) | (89) |
| acute: WAz; chronic: HAz | United States, Single-center | Children, PRIMS III 5.0 (range 0–29); (N=240) | chronic: 23.3% | (90) |
| BMIz | 8 countries, 31 PICUs | MV children, 4.5 years (SD 5.1), severe severity of illness: 28.2%; (N=500) | 17.1%; mild: 14.4% | (91) |
| <2 years: WAz, HAz, WHz; >2 years: BMIz; chronic diseases: HAz | Brazil, Single-center | Children, 18.3 months [IQR 3.9–63.3], PIM2 2.0 [IQR 1–5.9] (N=385) | 45.5% | (92) |
| <2 years: WAz; >2 years: BMIz | Brazil, Single center | Children receiving EN, 11 months [IQR 3–65], PIM2 11 [IQR 3–65]; (N=291) | 41.2% | (93) |
| WAz, HAz, WHz, BMIz, MUACz | Brazil, Single-center | Children with PICU LOS > 7 days, <2 years: 41%; (N=90) | WAz: 27.7%, HAz: 50%, WHz: 8%, BMIz: 13.3%, MUACz: 47.8% | (51) |
| WAz | Canada, Single-center | MV children, 0.65 years [IQR 0.17–6.2], PIM2 6.8 [4.9–10.5]; (N=49) | 20% | (94) |
| <2 years: WAz; >2 years: BMIz | Brazil, Single-center | Children, 1.8 years [IQR 0.43–7.17], PIM2 1.45 [IQR 0.85–4.95]. N=82) | 39.1% | (95) |
| WAz | Brazil, Single-center | Children, undernourished 25.6 months / well-nourished 10.7 months; (N=1077) | 53% | (96) |
| acute: WAz, MUACz; chronic: HAz, TSFz | Netherlands, Single-center | Children, >30 days: 32%, 1.4 years (range 31 days–17 years), PRISM 11 (range 0–38); (N=293) | In children > 30 days; acute: WAz: 24%; MUACz: 15%; chronic: HAz: 22%; TSFz: 10% | (38) |
| Others Cut offs | ||||
| BMI < P5 | United States, 53 PICUs | Children with sepsis and septic shock; (N=7038) | 11.6% | (97) |
| <2 years: WHz < P5; >2 years: BMIz <-1.89 or < P3 | United States, Single-center | Children, <1 year: 24.8%; (N=1447) | 15% | (98) |
| <2 years: WA percentile; > 2 years: BMI percentile | Egypt, Single-center | Children, 14.3 months (SD 13.9); (N=50) | 24% | (99) |
| BMI-z < -1.89 | 43 PICUs, (5 studies) | Children older than 2 years with PARDS, 9.6 years (SD 5.1), PRISM3 11 [IQR 6–16]; (N=331). | 12% | (100) |
| <2 years: WH <P5; >2 years: BMI <P5 | Canada, Single-center | Children, 17 months [IQR 5–51], PRISM3 4 [IQR 1.8–9] (N=100) | 21% | (101) |
| WA <P3; acute: W/P50 WA<0.85 | Spain, Single-center | Children with AKI receiving continuous renal replacement therapy, 18.5 months [IQR 4.0–81.8], PRISM score 13 [IQR 10–20]; (N=174) | 35%; acute: 56% | (102) |
| acute: W/P50 WH <80%; chronic: H/P50 HA <90% | United States, Single-center | Children, 80 months, PRISM 8.89 (SE 0.76); (N=37) | acute: 8.1%; chronic: 22.1% | (8) |
| WA< P10; severe: WA< P3 | Spain, Single-center | MV children, 7.5 months [IQR 3.8–25.8], PIM2 3.7 [IQR 2.2–9.5]; (N=46) | 71.7%; severe: 58.7% | (9) |
| acute: W/P50 WH <80%; chronic: H/P50 HA <90% | Greece, Single-center | MV children, 71.8 (SEM 6.8) months, PRISM 12.8 (SEM 0.84); (N=71) | acute: 5.6%; chronic: 4.2% | (103) |
N, number of participants; BMI, body mass index; W, weight; H, height; HA, height-for-age; WA, weight-for-age; MUAC, mid-upper arm circumference; TSF, triceps skinfold; W / P50 WH, actual weight/ weight-for-height in 50th percentile ratio; SEM, standard error of mean; IQR, interquartile range; SD, standard deviation. WHz, weight-for-length z score; P, percentile; PIM2, pediatric index of mortality 2; PRISM, pediatric risk of mortality; CHD, congenital heart disease; AKI, acute kidney injury; PICU, pediatric intensive care unit; LOS, length of stay; MV, mechanical ventilation; PARDS, pediatric acute respiratory distress syndrome; EN, enteral nutrition. †, AKI: injury + failure.
The differences in the prevalence of undernutrition can be attributed to the large variability in ages, severity of the disease, presence of complex chronic conditions, and PICU settings and countries. The main risk factors associated with undernutrition at admission were younger age and underlying chronic condition (37,98,100).
NS deterioration during PICU stay
Table 2 describes the NS deterioration during PICU LOS. Few studies have described the NS changes during PICU stay, and there is a lack of standardization of the cut-off points. Therefore, it is not possible to estimate the real prevalence of NS deterioration during critical illness in children. A French single-center prospective study found that in children who stayed longer than 5 days, PICU-acquired faltering growth was observed to be an early, significant, and relatively frequent phenomenon (37).
Table 2
| Parameters | Setting | Population | NS Changes | Reference |
|---|---|---|---|---|
| PICU-acquired faltering growth, BMIz | France, Single-center | Children, 13.6 months [IQR 1.9-96.1], PIM2 4.5 [1.5-8.5]; (N=579) | Faltering growth: 10.2%; |
(37) |
| Weight | Canada, Single-center | Children, 17 months [IQR 5-51], PRISM3 4 [IQR 1.8-9] (N=100) | <6 months: ↑ 3.0% of admission weight; |
(101) |
| WAz, HAz, WHz, BMIz, MUACz or TSF (mm) | Brazil, Single-center | Children with PICU LOS > 7 days, <2 years: 41%. (N=90) | In 7 days: ↓ MUACz and ↓ TSF (mm) | (51) |
| NS deterioration | Netherlands, Single-center | Children, 1.4 years (range 31 days-17years) in children older than 30 days, PRISM 11 (range 0–38); (N=293) | In children > 30 days: 4% with NS deterioration | (38) |
N, number of participants; BMI, body mass index; HA, height-for-age; WA, weight-for-age; MUAC, mid-upper arm circumference; TSF, triceps skinfold; IQR, interquartile range; SD, standard deviation. WHz, weight-for-length z score; pediatric index of mortality 2; PRISM, pediatric risk of mortality; PICU, pediatric intensive care unit. PICU-acquired faltering growth, decline of 1 standard deviation in BMIz. NS deterioration, decline of 1 standard deviation in weight-for-age z-score.
Consequences of undernutrition during critical illness
Short-term outcomes
Table 3 describes the association of undernutrition during critical illness and outcomes in children. Undernutrition at PICU admission, based on a single anthropometric measurement, is associated to adverse clinical outcomes, such as increased mortality (65,78,102), increased duration of mechanical ventilation (60,76,78,92), increased PICU LOS (60,65,98), and hospital LOS (78,98). Although undernutrition based on a single anthropometric measurement is associated to adverse clinical outcomes, it is important to highlight that single anthropometric measurement does not evaluate important aspects of NS, such as growth velocity, chronicity, and functional status (6,25).
Table 3
| Undernutrition | Setting | Population | Main Results | Reference |
|---|---|---|---|---|
| WAz, HAz, BMIz, WHz or MUACz: < –2 z score | China, Single-center | Pediatric oncology, 58 months [IQR 1–205], severe critical illness: 26.25%. (N=160) | • WAz, HAz, WHz and MUACz: ↑ duration of MV | (60) |
| • WAz: ↑ PICU LOS | ||||
| (adjusted by sex, age, and pediatric critical illness score) | ||||
| WHz: < –2 z score | Singapore Single-center | Children with severe bronchiolitis, 4.9 months [IQR 2–10.4], 62.2% with multiorgan dysfunction. (N=74) | • ↑ duration of MV | (63) |
| (multivariate analysis SpO2 to FiO2 ratio, multiorgan dysfunction and baseline comorbidities) | ||||
| BMIz, MUACz or TSFz: < –2 z score | Brazil, Single-center | Children, 23.1 months [IQR 3.9–89.1], PIM2 4.2 [IQR 1.3–15.7]. (N=199) | • BMIz, MUACz or TSFz: ↑ 60-day mortality | (65) |
| • MUACz: ↑ PICU LOS | ||||
| (adjusted by sex, age, PIM2, and complex chronic condition) | ||||
| <2 years: WH <P5 >2 years: BMI <P5 | United States 53 PICUs | Children with sepsis and septic shock (N=7038) | No association with mortality | (97) |
| <2 years: WHz < P5 >2 years: BMIz < –1.89 or < P3 | United States, Single-center | Children, <1 year: 24.8%. (N=1447) | • ↑ PICU LOS (in comparison to normal or obese) | (98) |
| • ↑ Hospital LOS | ||||
| (adjusted) | ||||
| <2 years: WHz >2 years: BMIz | United States, Single-center | MV children, 9.5 months, PRISM3 ≥10: 26.4%. (N=106) | No association with any worse clinical outcome measure (duration MV, PICU LOS and hospital LOS) | (72) |
| BMIz < –2 z score | 26 countries, 129 PICUs | Children with severe sepsis, <12 months: 37%. (N=417) | • BMIz < –3: ↑ all-cause ICU mortality | (73) |
| (adjusted) | ||||
| Faltering growth | France, Single-center | Children, 18.8 months [IQR 2.7–103.8], PIM2 2 [IQR 1–6]. (N=683) | • ↑ PICU LOS | (26) |
| (adjusted by age, sex, chronic underlying disease, acquired infection, and PIM2 score) | ||||
| WAz < –2 z score | Taiwan, Single-center | Children, 4.4 years (range 0.1–18). (N=282) | • WAz ≤–2 and PIM2: ↑ morbidity (overall poor outcomes) | (75) |
| (adjusted by age, PIM2, hypotension, noninfectious diseases, creatinine sex) | ||||
| WAz, HAz, BMIz, UAMAz < –2 z score | Brazil, Single-center | MV children, 21.1 months [IQR 4.4–82.2], PIM2 8.0 [IQR 1.6–21.1]. (N=72) | • WAz, HAz or UAMAz: ↑ duration of MV | (76) |
| (adjusted by sex, age, PIM2) | ||||
| BMIz < –2 z score | 16 countries, 90 PICUs | MV children, 4.5 years (SD 5.1), severe severity of illness: 29%. (N=1622) | • BMIz: ↑ 60-day mortality†, ↑ acquired infections‡, ↓ VFD, hospital LOS‡ | (78) |
| (adjusted by: †location, age, sex, study year, admission category, diagnosis category, PICU size, infections, LOS, and illness severity, ‡age, sex, study year, admission category, diagnosis category, illness severity, and PICU size) | ||||
| BMI-z < –1.89 | International43 PICUs | Children older than 2 years with PARDS, 9.6 years (SD 5.1), PRISM–III score 11 [IQR 6–16]. (N=331). | No association with mortality | (100) |
| <2 years: WAz/HAz/WHz < –2 z score >2 years: BMIz < –2 z score chronic diseases: HAz < –2 z score | Brazil, Single-center | Children, 18.3 months [IQR 3.9–63.3], PIM2 2.0 [IQR 1–5.9] (N=385) | • ↔ hospital LOS | (92) |
| • ↑ duration MV | ||||
| (adjusted by septic shock, PELOD>11, medical diagnose) |
N, number of participants; BMIz, body mass index-for-age z score; HAz, height-for-age z score; WAz, weight-for-age z score; WHz, weight-for-length z score; MUACz, mid-upper arm circumference-for-age z score; UAMAz, upper arm muscle area-for-age z-score; TSF, triceps skinfold; IQR, interquartile range; P, percentile; PIM2, pediatric index of mortality 2; PRISM, pediatric risk of mortality; PELOD, pediatric logistic organ dysfunction; PICU, pediatric intensive care unit; LOS: length of stay; MV, mechanical ventilation; VFD, ventilator free days. Faltering growth: decline of 1 standard deviation in WAz.
Additionally, NS deterioration may have an impact on PICU outcomes. However, there are limited data available on the NS change during PICU stay, and the intensity, frequency, and impact of NS deterioration on adverse outcomes are still uncertain (37).
Long term outcomes
Besides the effects during PICU stay, undernutrition in critical illness and the PICU experience itself, may also adversely affect overall long-term pediatric outcomes, such as the development and growth, in addition to other relevant outcomes (82). The increase in the number of survivors observed in the last few decades leads to a higher number of patients using and needing post-intensive care health services (82,85).
It is important to highlight that evaluating morbidity only at discharge may underestimate the consequences associated with a critical illness. Conditions present before the admission or acquired during PICU stay may progress after discharge, increasing morbidity and mortality of children previously admitted to a PICU (105). It is known from adult studies that the impact of the negative protein balance on muscle function and quality of life can continue for months or years (106). Although some studies showed a recovery on NS after 3 months (26) to 6 months (38) of PICU discharge, the influence of undernutrition, NS deterioration, or muscle wasting during the critical illness on long-term outcomes is still unknown.
Conclusions
The identification of undernutrition in critically ill children is important since they are most vulnerable and therefore potentially may benefit from timely nutrition intervention (11). In critically ill children, moderate to severe undernutrition is prevalent at PICU admission worldwide, and it is associated with worsened clinical outcomes. However, the prevalence of children at nutritional risk is unknown. Also, there are not NSTs validated for critically ill children. Considering that in a limited resource setting, timely and detailed nutrition assessment of every child in the PICU may not be feasible (11), efforts should be driven to the development of NSTs feasible to apply in PICU.
Moreover, there are limited data regarding NS deterioration during PICU LOS. The definition of NS deterioration and studies to evaluate the association of NS deterioration during pediatric critical illness and their impact on short-term and long-term outcomes are needed.
Acknowledgments
Funding: Oliveira LDA, Ventura JC and Silveira, TT received a scholarship provided by the Coordination for the Improvement of Higher Education Personnel (CAPES).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lyvonne Tume, Frederic Valla and Sascha Verbruggen) for the series “Nutrition in the Critically Ill Child” published in Pediatric Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm-20-66). The series “Nutrition in the Critically Ill Child” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- United Nations Children’s Fund, World Health Organization, World Bank Group. Levels and trends in child malnutrition: key findings of the 2015 edition. World Health Organization 2015;1-6.
- Kyu HH, Pinho C, Wagner JA, et al. Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013. JAMA Pediatr 2016;170:267-87. [Crossref] [PubMed]
- McCarthy A, Delvin E, Marcil V, et al. Prevalence of Malnutrition in Pediatric Hospitals in Developed and In-Transition Countries: The Impact of Hospital Practices. Nutrients 2019;11:236. [Crossref] [PubMed]
- Kittisakmontri K, Sukhosa O. The financial burden of malnutrition in hospitalized pediatric patients under five years of age. Clin Nutr ESPEN 2016;15:38-43. [Crossref] [PubMed]
- Abdelhadi RA, Bouma S, Bairdain S, et al. Characteristics of Hospitalized Children With a Diagnosis of Malnutrition. JPEN J Parenter Enteral Nutr 2016;40:623-35. [Crossref] [PubMed]
- Mehta NM, Corkins MR, Lyman B, et al. Defining pediatric malnutrition: A paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr 2013;37:460-81. [Crossref] [PubMed]
- Becker P, Carney LN, Corkins MR, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: indicators recommended for the identification and documentation of pediatric malnutrition (undernutrition). Nutr Clin Pract 2015;30:147-61. [Crossref] [PubMed]
- Briassoulis G, Venkataraman S, Thompson A. Nutritional-metabolic factors affecting nitrogen balance and substrate utilization in the critically ill. J Pediatr Intensive Care 2012;1:77-86. [PubMed]
- Botrán M, López-Herce J, Mencía S, et al. Relationship between energy expenditure, nutritional status and clinical severity before starting enteral nutrition in critically ill children. Br J Nutr 2011;105:731-7. [Crossref] [PubMed]
- Martinez EE, Mehta NM. The science and art of pediatric critical care nutrition. Curr Opin Crit Care 2016;22:316-24. [Crossref] [PubMed]
- Mehta NM, Skillman HE, Irving SY, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr 2017;41:706-42. [Crossref] [PubMed]
- Joosten K, van Puffelen E, Verbruggen S. Optimal nutrition in the paediatric ICU. Curr Opin Clin Nutr Metab Care 2016;19:131-7. [Crossref] [PubMed]
- Mehta NM, Duggan CP. Nutritional Deficiencies During Critical Illness. Pediatr Clin North Am 2009;56:1143-60. [Crossref] [PubMed]
- De Cosmi V, Milani GP, Mazzocchi A, et al. The Metabolic Response to Stress and Infection in Critically Ill Children: The Opportunity of an Individualized Approach. Nutrients 2017;9:1032. [Crossref] [PubMed]
- Joosten KFM, Eveleens RD, Verbruggen SCAT. Nutritional support in the recovery phase of critically ill children. Curr Opin Clin Nutr Metab Care 2019;22:152-8. [Crossref] [PubMed]
- Preiser JC, Ichai C, Orban JC, et al. Metabolic response to the stress of critical illness. Br J Anaesth 2014;113:945-54. [Crossref] [PubMed]
- Mehta NM. Nutritional Status and Outcomes in Pediatric Severe Sepsis-Size Matters. Crit Care Med 2018;46:1886-7. [Crossref] [PubMed]
- Marik PE. Feeding critically ill patients the right “whey”: thinking outside of the box. A personal view. Ann Intensive Care 2015;5:51. [Crossref] [PubMed]
- Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 2015;14:58-74. [Crossref] [PubMed]
- Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591-600. [Crossref] [PubMed]
- Moore FA, Phillips SM, McClain CJ, et al. Nutrition Support for Persistent Inflammation, Immunosuppression, and Catabolism Syndrome. Nutr Clin Pract 2017;32:121S-7S. [Crossref] [PubMed]
- Kantor DB, Mehta NM. Ultrasound Measurement of Muscle Thickness: A Novel Biomarker for the PICU? Pediatr Crit Care Med 2017;18:817-9. [Crossref] [PubMed]
- Valla FV, Ford-Chessel C, Meyer R, et al. A training program for anthropometric measurements by a dedicated nutrition support team improves nutritional status assessment of the critically ill child. Pediatr Crit Care Med 2015;16:e82-8. [Crossref] [PubMed]
- Prieto MB, Cid JLH. Malnutrition in the critically ill child: the importance of enteral nutrition. Int J Environ Res Public Health 2011;8:4353-66. [Crossref] [PubMed]
- Tume LN, Valla FV, Floh AA, et al. Priorities for Nutrition Research in Pediatric Critical Care. JPEN J Parenter Enteral Nutr 2019;43:853-62. [Crossref] [PubMed]
- Valla FV, Berthiller J, Gaillard-Le-Roux B, et al. Faltering growth in the critically ill child: prevalence, risk factors, and impaired outcome. Eur J Pediatr 2018;177:345-53. [Crossref] [PubMed]
- Hulst JM, Huysentruyt K, Joosten KF. Pediatric screening tools for malnutrition: an update. Curr Opin Clin Nutr Metab Care 2020;23:203-9. [Crossref] [PubMed]
- Hulst JM, Zwart H, Hop WC, Joosten KF. Dutch national survey to test the STRONGkids nutritional risk screening tool in hospitalized children. Clin Nutr 2010;29:106-11. [Crossref] [PubMed]
- Gerasimidis K, Macleod I, Maclean A, et al. Performance of the novel Paediatric Yorkhill Malnutrition Score (PYMS) in hospital practice. Clin Nutr 2011;30:430-5. [Crossref] [PubMed]
- Gerasimidis K, Keane O, MacLeod I, et al. A four-stage evaluation of the Paediatric Yorkhill Malnutrition Score in a tertiary paediatric hospital and a district general hospital. Br J Nutr 2010;104:751-6. [Crossref] [PubMed]
- Lima da Silva JCG, Raposo Miranda S, Vieira de Melo CYS. Preoperative nutritional risk through total lymphocyte content, serum albumine levels and strongkids tribal tool in children submitted to cardiac surgeries. Nutr clínica y dietética Hosp 2019;39:50-7.
- Qiao JY, Guo FF, Li F, Chen LX, Luan B. Nutritional assessment and clinical application of nutritional risk screening tools in critically ill children. al title should be Zhongguo Dang Dai Er Ke Za Zhi 2019;21:528-33. [PubMed]
- Aziz MM, Haroun M, Gad OM. STRONGKIDS Nutritional risk score and Body Mass Index in Malnourishment Risk assessment in Critically Ill Children. Int J Pharm Phytopharm Res 2019;9:97-108.
- Tume LN, Valla FV, Joosten K, et al. Nutritional support for children during critical illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations. Intensive Care Med 2020;46:411-25. [Crossref] [PubMed]
- Irving SY, Seiple S, Nagle M, et al. Perceived barriers to anthropometric measurements in critically ill children. Am J Crit Care 2015;24:e99-107. [Crossref] [PubMed]
- Mehta NM, Compher C A.S.P.E.N.. Clinical Guidelines: nutrition support of the critically ill child. JPEN J Parenter Enteral Nutr 2009;33:260-76. [Crossref] [PubMed]
- Valla FV, Baudin F, Gaillard Le Roux B, et al. Nutritional Status Deterioration Occurs Frequently During Children’s ICU Stay. Pediatr Crit Care Med 2019;20:714-21. [Crossref] [PubMed]
- Hulst J, Joosten K, Zimmermann L, et al. Malnutrition in critically ill children: from admission to 6 months after discharge. Clin Nutr 2004;23:223-32. [Crossref] [PubMed]
- Keller U. Nutritional Laboratory Markers in Malnutrition. J Clin Med 2019;8:775. [Crossref] [PubMed]
- Tekgüç H, Özel D, Sanaldi H, et al. Prealbumin and Retinol Binding Proteins Are Not Usable for Nutrition Follow-Up in Pediatric Intensive Care Units. Pediatr Gastroenterol Hepatol Nutr 2018;21:321. [Crossref] [PubMed]
- Bharadwaj S, Ginoya S, Tandon P, et al. Malnutrition: Laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf) 2016;4:272-80. [PubMed]
- Tiwari LK, Singhi S, Jayashree M, et al. Hypoalbuminemia in critically sick children. Indian J Crit Care Med 2014;18:565-9. [Crossref] [PubMed]
- Kittisakmontri K, Reungrongrat S, Lao-araya M. Hypoalbuminaemia at admission predicts the poor outcomes in critically ill children. Anaesthesiol Intensive Ther 2016;48:158-61. [Crossref] [PubMed]
- Leite HP, Rodrigues da Silva AV, de Oliveira Iglesias SB, et al. Serum Albumin Is an Independent Predictor of Clinical Outcomes in Critically Ill Children. Pediatr Crit Care Med 2016;17:e50-7. [Crossref] [PubMed]
- Bhandarkar N, Save S, Bavdekar SB, et al. Serum Albumin and C-Reactive Protein as Predictors of Adverse Outcomes in Critically Ill Children: A Prospective Observational Pilot Study. Indian J Pediatr 2019;86:758-9. [Crossref] [PubMed]
- Dao DT, Anez-Bustillos L, Cho BS, et al. Assessment of Micronutrient Status in Critically Ill Children: Challenges and Opportunities. Nutrients 2017;9:1185. [Crossref] [PubMed]
- Marino LV, Valla FV, Beattie RM, et al. Micronutrient status during paediatric critical illness: A scoping review. Clin Nutr 2020;S0261-5614(20)30186-2.
- Valla FV, Bost M, Roche S, et al. Multiple Micronutrient Plasma Level Changes Are Related to Oxidative Stress Intensity in Critically Ill Children. Pediatr Crit Care Med 2018;19:e455-63. [Crossref] [PubMed]
- Bouma S. Diagnosing Pediatric Malnutrition: Paradigm Shifts of Etiology-Related Definitions and Appraisal of the Indicators. Nutr Clin Pract 2017;32:52-67. [Crossref] [PubMed]
- Eskedal LT, Hagemo PS, Seem E, et al. Impaired weight gain predicts risk of late death after surgery for congenital heart defects. Arch Dis Child 2008;93:495-501. [Crossref] [PubMed]
- Zamberlan P, Delgado AF, Leone C, et al. Nutrition therapy in a pediatric intensive care unit: indications, monitoring, and complications. JPEN J Parenter Enteral Nutr 2011;35:523-9. [Crossref] [PubMed]
- Kelleher DK, Laussen P, Teixeira-Pinto A, et al. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition 2006;22:237-44. [Crossref] [PubMed]
- van Puffelen E, Hulst JM, Vanhorebeek I, et al. Effect of late versus early initiation of parenteral nutrition on weight deterioration during PICU stay: Secondary analysis of the PEPaNIC randomised controlled trial. Clin Nutr 2020;39:104-9. [Crossref] [PubMed]
- Liebau F, Norberg Å, Rooyackers O. Does feeding induce maximal stimulation of protein balance? Curr Opin Clin Nutr Metab Care 2016;19:120-4. [Crossref] [PubMed]
- Johnson RW, Ng KWP, Dietz AR, et al. Muscle atrophy in mechanically-ventilated critically ill children. PLoS One 2018;13:e0207720 [Crossref] [PubMed]
- Valla FV, Young DK, Rabilloud M, et al. Thigh ultrasound monitoring identifies decreases in quadriceps femoris thickness as a frequent observation in critically ill children. Pediatr Crit Care Med 2017;18:e339-47. [Crossref] [PubMed]
- Ong C, Lee JH, Leow MKS, et al. Skeletal Muscle Ultrasonography in Nutrition and Functional Outcome Assessment of Critically Ill Children: Experience and Insights from Pediatric Disease and Adult Critical Care Studies. PEN J Parenter Enteral Nutr 2017;41:1091-9. [Crossref] [PubMed]
- Martinez EE, Smallwood CD, Quinn NL, et al. Body Composition in Children with Chronic Illness: Accuracy of Bedside Assessment Techniques. J Pediatr 2017;190:56-62. [Crossref] [PubMed]
- Fullerton BS, Sparks EA, Khan FA, et al. Whole Body Protein Turnover and Net Protein Balance After Pediatric Thoracic Surgery: A Noninvasive Single-Dose 15 N Glycine Stable Isotope Protocol With End-Product Enrichment. PEN J Parenter Enteral Nutr 2018;42:361-70. [PubMed]
- Feng S, Cheng L, Lu H, et al. Nutritional Status and Clinical Outcomes in Children with Cancer on Admission to Intensive Care Units. Nutr Cancer 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Jhang WK, Park SJ. Energy Expenditure in Mechanically Ventilated Korean Children. Pediatr Crit Care Med 2020;21:e522-9. [Crossref] [PubMed]
- Melro EC, de Souza Lima AE, Missagia de Mattos Springer A, et al. Protein intake deficiency in critically ill children with respiratory insufficiency: A call to action? Clin Nutr ESPEN 2020;37:69-74. [Crossref] [PubMed]
- Ng GYH, Ong C, Wong JJM, et al. Nutritional status, intake, and outcomes in critically ill children with bronchiolitis. Pediatr Pulmonol 2020;55:1199-206. [Crossref] [PubMed]
- Srinivasan V, Hasbani NR, Mehta NM, et al. Early Enteral Nutrition Is Associated With Improved Clinical Outcomes in Critically Ill Children. Pediatr Crit Care Med 2020;21:213-21. [Crossref] [PubMed]
- Ventura JC, Hauschild DB, Barbosa E, et al. Undernutrition at PICU Admission Is Predictor of 60-Day Mortality and PICU Length of Stay in Critically Ill Children. J Acad Nutr Diet 2020;120:219-29. [Crossref] [PubMed]
- Goday PS, Kuhn EM, Mikhailov TA. Early Parenteral Nutrition in Critically Ill Children Not Receiving Early Enteral Nutrition Is Associated With Significantly Higher Mortality. JPEN J Parenter Enter Nutr. JPEN J Parenter Enteral Nutr 2020;44:1096-103. [Crossref] [PubMed]
- Jacquot A, Valla FV, Mura T, et al. NUTRI-REAPED study: nutritional assessment of French critically ill children and nutrition practice survey in French-speaking pediatric intensive care units. Ann Intensive Care 2019;9:15. [Crossref] [PubMed]
- Singh M, Sankar J, Kumar A, et al. Predictors of Mortality in Children Admitted to the Pediatric Intensive Care Unit with Acute Gastroenteritis with Severe Dehydration. Indian J Pediatr 2019;86:1142-5. [Crossref] [PubMed]
- Marino LV, Eveleens RD, Morton K, et al. Peptide nutrient-energy dense enteral feeding in critically ill infants: an observational study. J Hum Nutr Diet 2019;32:400-8. [Crossref] [PubMed]
- Zaher S, White D, Ridout J, et al. Association between enteral macronutrient delivery and inflammatory response in critically ill children. Clin Nutr 2019;38:2287-96. [Crossref] [PubMed]
- Zamberlan P, Feferbaum R, Doria Filho U, et al. Bioelectrical Impedance Phase Angle and Morbidity and Mortality in Critically Ill Children. Nutr Clin Pract 2019;34:163-71. [Crossref] [PubMed]
- Haney A, Burritt E, Babbitt CJ. The impact of early enteral nutrition on pediatric acute respiratory failure. Clin Nutr ESPEN 2018;26:42-6. [Crossref] [PubMed]
- Irving SY, Daly B, Verger J, et al. The association of nutrition status expressed as body mass index z score with outcomes in children with severe sepsis: A secondary analysis from the sepsis prevalence, outcomes, and therapies (SPROUT) study. Crit Care Med 2018;46:e1029-39. [Crossref] [PubMed]
- Velazco CS, Zurakowski D, Fullerton BS, et al. Nutrient delivery in mechanically ventilated surgical patients in the pediatric critical care unit. J Pediatr Surg 2017;52:145-8. [Crossref] [PubMed]
- Chen MY, Yang YJ. Being Underweight Is an Independent Risk Factor for Poor Outcomes Among Acutely Critically Ill Children. Nutr Clin Pract 2018;33:433-8. [PubMed]
- Grippa RB, Silva PS, Barbosa E, et al. Nutritional status as a predictor of duration of mechanical ventilation in critically ill children. Nutrition 2017;33:91-5. [Crossref] [PubMed]
- Nyirasafari R, Corden MH, Karambizi AC, et al. Predictors of mortality in a paediatric intensive care unit in Kigali, Rwanda. Paediatr Int Child Health 2017;37:109-15. [Crossref] [PubMed]
- Bechard LJ, Duggan C, Touger-Decker R, et al. Nutritional Status Based on Body Mass Index Is Associated With Morbidity and Mortality in Mechanically Ventilated Critically Ill Children in the PICU. Crit Care Med 2016;44:1530-7. [Crossref] [PubMed]
- Manzoli TF, Delgado AF, Troster EJ, et al. Lymphocyte count as a sign of immunoparalysis and its correlation with nutritional status in pediatric intensive care patients with sepsis: A pilot study. Clinics 2016;71:644-9. [Crossref] [PubMed]
- Bagri NK, Jose B, Shah SK, et al. Impact of Malnutrition on the Outcome of Critically Ill Children. Indian J Pediatr 2015;82:601-5. [Crossref] [PubMed]
- de Betue CTI, van Steenselen WN, Hulst JM, et al. Achieving energy goals at day 4 after admission in critically ill children; predictive for outcome? Clin Nutr 2015;34:115-22. [Crossref] [PubMed]
- Ekim A. The post-intensive care syndrome in children. Compr Child Adolesc Nurs 2020;43:15-21. [Crossref] [PubMed]
- Mara J, Gentles E, Alfheeaid HA, et al. An evaluation of enteral nutrition practices and nutritional provision in children during the entire length of stay in critical care. BMC Pediatr 2014;14:186. [Crossref] [PubMed]
- Mikhailov TA, Kuhn EM, Manzi J, et al. Early enteral nutrition is associated with lower mortality in critically ill children. JPEN J Parenter Enteral Nutr 2014;38:459-66. [Crossref] [PubMed]
- Manning JC, Pinto NP, Rennick JE, et al. Conceptualizing post intensive care syndrome in children - The PICS-p framework. Pediatr Crit Care Med 2018;19:298-300. [Crossref] [PubMed]
- Abdul Manaf Z, Kassim N, Hamzaid NH, et al. Delivery of enteral nutrition for critically ill children. Nutr Diet 2013;70:120-5. [Crossref]
- Leite HP, de Lima LFP, de Oliveira Iglesias SB, et al. Malnutrition may worsen the prognosis of critically ill children with hyperglycemia and hypoglycemia. JPEN J Parenter Enteral Nutr 2013;37:335-41. [Crossref] [PubMed]
- de Menezes FS, Leite HP, Nogueira PC. What are the factors that influence the attainment of satisfactory energy intake in pediatric intensive care unit patients receiving enteral or parenteral nutrition? Nutrition 2013;29:76-80. [Crossref] [PubMed]
- Kyle UG, Akcan-Arikan A, Orellana RA, et al. Nutrition support among critically ill children with AKI. Clin J Am Soc Nephrol 2013;8:568-74. [Crossref] [PubMed]
- Kyle UG, Jaimon N, Coss-Bu JA. Nutrition Support in Critically Ill Children: Underdelivery of Energy and Protein Compared with Current Recommendations. J Acad Nutr Diet 2012;112:1987-92. [Crossref] [PubMed]
- Mehta NM, Bechard LJ, Cahill N, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children--an international multicenter cohort study. Crit Care Med 2012;40:2204-11. [Crossref] [PubMed]
- de Souza Menezes F, Leite HP, Koch Nogueira PC. Malnutrition as an independent predictor of clinical outcome in critically illchildren. Nutrition 2012;28:267-70. [Crossref] [PubMed]
- Vidigal MV, Leite HP, Nogueira PC. Factors associated with peptide-based formula prescription in a pediatric intensive care unit. J Pediatr Gastroenterol Nutr 2012;54:620-3. [Crossref] [PubMed]
- Bockenkamp B, Jouvet P, Arsenault V, et al. Assessment of calories prescribed and delivered to critically ill children. E Spen Eur E J Clin Nutr Metab 2009;4:e172-5. [Crossref]
- Santana e Meneses JF, Leite HP, de Carvalho WB, et al. Hypophosphatemia in critically ill children: Prevalence and associated risk factors. Pediatr Crit Care Med 2009;10:234-8. [Crossref] [PubMed]
- Delgado AF, Okay TS, Leone C, et al. Hospital malnutrition and inflammatory response in critically ill children and adolescents admitted to a tertiary intensive care unit. Clinics (Sao Paulo) 2008;63:357-62. [Crossref] [PubMed]
- Ross PA, Klein MJ, Nguyen T, et al. Body Habitus and Risk of Mortality in Pediatric Sepsis and Septic Shock: A Retrospective Cohort Study. J Pediatr 2019;210:178-83.e2. [Crossref] [PubMed]
- Sharma K, Raszynski A, Totapally BR. The impact of body mass index on resource utilization and outcomes of children admitted to a pediatric intensive care unit. SAGE Open Med 2019;7:2050312119825509 [Crossref] [PubMed]
- El Shazly AN, Soliman DR, Assar EH, et al. Phosphate disturbance in critically ill children: Incidence, associated risk factors and clinical outcomes. Ann Med Surg (Lond) 2017;21:118-23. [Crossref] [PubMed]
- Ward SL, Gildengorin V, Valentine SL, et al. Impact of Weight Extremes on Clinical Outcomes in Pediatric Acute Respiratory Distress Syndrome. Crit Care Med 2016;44:2052-9. [Crossref] [PubMed]
- Keehn A, O’Brien C, Mazurak V, et al. Epidemiology of Interruptions to Nutrition Support in Critically Ill Children in the Pediatric Intensive Care Unit. JPEN J Parenter Enteral Nutr 2015;39:211-7. [Crossref] [PubMed]
- Castillo A, Santiago MJ, López-Herce J, et al. Nutritional status and clinical outcome of children on continuous renal replacement therapy: a prospective observational study. BMC Nephrol 2012;13:125. [Crossref] [PubMed]
- Briassoulis G, Zavras N, Hatzis T. Malnutrition, nutritional indices, and early enteral feeding in critically ill children. Nutrition 2001;17:548-57. [Crossref] [PubMed]
- Mehta NM, Bechard LJ, Zurakowski D, et al. Adequate enteral protein intake is inversely associated with 60-d mortality in critically ill children : a multicenter, prospective, cohort study. Am J Clin Nutr 2015;102:199-206. [Crossref] [PubMed]
- Pinto NP, Rhinesmith EW, Kim TY, et al. Long-Term Function after Pediatric Critical Illness: Results from the Survivor Outcomes Study. Pediatr Crit Care Med 2017;18:e122-30. [Crossref] [PubMed]
- van Gassel RJJ, Baggerman MR, van de Poll MCG. Metabolic aspects of muscle wasting during critical illness. Curr Opin Clin Nutr Metab Care 2020;23:96-101. [Crossref] [PubMed]
Cite this article as: Moreno YMF, Ventura JC, de Almeida Oliveira LD, Silveira TT, Hauschild DB. Undernutrition in critically ill children. Pediatr Med 2020;3:22.

