
The biology of bilirubin production: overview of detection and inhibition
Introduction
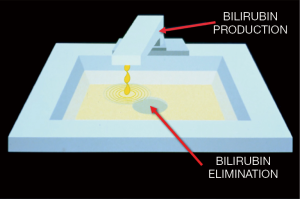
Neonatal jaundice is a syndrome arising from many different causes, but it can be easily understood by an analogy to a sink (Figure 1) (1). The turned-on spigot represents the process of bilirubin production and the drain represents the process of bilirubin elimination. The volume of the sink represents the capacity of the circulation to store bilirubin, which is determined primarily by the amount of albumin, the main binding protein for bilirubin, in the blood. Thus, if the rate of bilirubin production exceeds the rate of bilirubin elimination, then the level of total serum/plasma bilirubin (TB) in the circulation begins to rise (1,2). Moreover, when the ability of albumin to bind bilirubin is exceeded, the sink begins to “overflow”—representing bilirubin moving from the circulation into tissues. This latter phenomenon becomes manifest in the skin and conjunctiva as “jaundice,” but also occurs in other tissues less easily seen with the naked eye, for example, the brain. The accumulation of bilirubin in discrete regions of the brain (i.e., globus pallidus) can lead to the syndrome of bilirubin-induced neurologic dysfunction (BIND) (3-5) and to acute or irreversible—known as “acute bilirubin encephalopathy (ABE)”—or chronic or permanent [traditionally called “kernicterus” or now “chronic bilirubin encephalopathy (CBE)”] clinical manifestations (6).
Simply put, jaundice cannot occur without first, the production of the pigment and second, the excessive production of the pigment relative to its elimination, as is the case in most neonates in the transitional period after birth (1,7). This circumstance is fundamental to the occurrence of all kinds of jaundice, physiologic or pathophysiologic. Most pathophysiologic jaundice requiring treatment is caused by increased bilirubin production rates (reflected as hyperbilirubinemia) above what is considered normal in all term newborns (approximately 2 to 3 times higher compared to an adult) (8), which is caused by a relatively large red cell mass and a shorter red blood cell (RBC) lifespan as a function of age-in-hours (9). In preterm infants, the RBC mass is slightly less than term infants, but the RBC lifespan is shorter resulting in corresponding higher bilirubin production rates on a body weight basis (1,10). Thus, the traditional epidemiologic risk factors for neonatal jaundice are easily understood, such as hemolysis, bruising, closed-space bleeding, and polycythemia, as they all contribute to an increase in heme catabolism and thus increased production of bilirubin (1,2,7). Of course, in all newborns, the conjugation of bilirubin is transiently impaired (and again even more immature in preterm infants), and together with any genetic polymorphisms that contribute to impairment of uridine 5'-diphosho-glucuronosyltransferase (UGT1A1) activity (11,12), result in further impairment of bilirubin elimination and can further exacerbate the risk for neonatal jaundice in the context of increased bilirubin production rates.
The spigot in the analogy is the heme oxygenase (HO)-catalyzed step in the two-step process of heme catabolism (13). This reaction, which occurs in the endoplasmic reticulum, requires NADPH donated from the cytochrome P450 system and molecular oxygen and results in the equimolar production of carbon monoxide (CO), iron (Fe2+), and biliverdin, which is rapidly reduced in the cytosol to bilirubin. Because the reaction is ubiquitous and occurs in all nucleated cells, it would be impossible to know the total body production rate of bilirubin, except that CO is bound to hemoglobin, forming carboxyhemoglobin (COHb), is transported to the lungs and is continually excreted in breath (14,15). With certain assumptions about steady state, the CO excretion rate in breath, the end-tidal CO (ETCO) concentration, and the COHb concentration, when corrected for inhaled CO (ETCOc and COHbc, respectively) can each be used as indices of total body bilirubin production rates (2,16), making it possible to identify infants who are high producers of the pigment (2), and are therefore also at higher risk of neurologic injury (see below). To this end, various technologies and devices have been developed (2,16).
The association between increased bilirubin production and the risk for bilirubin neurotoxicity exists because babies with increased production of the pigment, for example due to hemolysis, are more likely to have greater amounts of bilirubin outside the circulation and in tissues as they exceed their bilirubin binding capacity (BBC) more rapidly (17-19). This phenomenon is often reflected in a rapid rate of rise in the circulating TB levels, and has been empirically taken by clinicians as a sign of increased bilirubin production (20,21). Moreover, two apparently identical TB levels can represent two very different situations of risk for neurologic injury, independent of the actual TB level (1). Consider the well, breastfeeding infant who reaches a TB level of 20 mg/dL at the end of the first week of life, and the hemolyzing infant who reaches the same level at 24 h of life (1). Because of individual variations in conjugating capacity among infants, not all high producers become hyperbilirubinemic to the point of requiring intervention, but most will at least become visibly jaundiced. However, high producers of the pigment, if they require treatment with phototherapy or exchange transfusion, are more likely to have rebound hyperbilirubinemia after treatment (22).
The most common treatment for neonatal hyperbilirubinemia is phototherapy, which was first suggested as a therapy in 1958 by Cremer (23). Of course, there are alternative therapeutic approaches, such as exchange transfusion, and other less common treatments. However, phototherapy is very effective with currently available devices, making other options less necessary today in most cases. It works because the bilirubin molecule interacts with certain wavelengths of light (peak absorbance at 478 nm) and undergoes photo-oxidation (a minor effect) and structural and configurational isomerization, the latter very rapidly (24,25). These products can be eliminated without conjugation, thus bypassing the temporary impairment in elimination because of immaturity or a genetic polymorphism affecting UGT1A1 activity (11,12). So what is the rationale for why inhibition of bilirubin production might be an alternative to phototherapy, at least in some cases?
In 2008, a paper described a randomized clinical trial of aggressive versus conservative phototherapy for infants with extremely low birthweight (ELBW) (26). There had not been a large trial of this sort since the large National Institutes of Health collaborative trial (27) reported on the efficacy of phototherapy to prevent exchange transfusion in predominately larger, more mature infants. However, in that trial, there was the suggestion that phototherapy might not be safe in the smallest babies, although the trial was not designed for making this determination. In the 2008 trial (26), the primary outcome was that aggressive phototherapy did not significantly reduce the rate of death or neurodevelopment impairment. Nonetheless, there were two planned secondary analyses: one showed that the rate of neurodevelopmental impairment alone was significantly reduced with aggressive phototherapy, confirming the importance of limiting the rise of TB levels in these infants; and the other showed that this reduction was offset by an increase in mortality among infants weighing 501 to 750 grams at birth, suggesting that there might be an adverse effect of using light. The paper raised the possibility that what Bill Silverman had described as “ambitious overgeneralization” had occurred in the case phototherapy (28). We, as a profession, had “overgeneralized” the use of phototherapy extending its application to ever smaller and more translucent patients for longer and longer timeframes without studying its safety. We should have asked the question sooner, “Is it possible that visible light has adverse effects in small premature infants?” The paper reinforced the need for reconsidering how best to apply phototherapy safely in these infants, resurrecting the notion of cycled phototherapy to reduce the dose of light as well as restrict the wavelengths of light to those known to be most effective with respect to interacting with the bilirubin molecule, namely narrow-wavelength blue light (29). But the paper also suggested a rationale for wanting to avoid phototherapy altogether in these very immature, antioxidant-deficient, small translucent patients (30,31), and consider a pharmacologic approach to control rising TB levels, such as inhibition of bilirubin production (32). Although controlling the spigot (Figure 1) would seem to be a conceptually simple and rational approach, it presents some challenges which are the subject of the subsequent discussion below.
Although the HO-mediated catabolic pathway for heme has been generally considered to be a source of potential toxins, including bilirubin, CO, and Fe2+, causing neurologic disturbances (33), mitochondrial dysfunction (34), and reactive oxygen species (ROS) production (35), respectively, it has many other roles in biology (33). For example, the biliverdin-bilirubin shunt has antioxidant, anti-inflammatory, and anti-apoptotic effects, and is important is maintaining the redox state of the cell (36). CO is an important signaling molecule in its own right, causing vessel relaxation through calcium and potassium dependent channels, as well through soluble guanylyl cyclase (sGC) and cyclic GMP, and mediating additional anti-platelet, anti-apoptotic (endothelial cells), anti-proliferative (vascular smooth muscle cells), and neurotransmission effects (37). CO also acts through p38MAPK to cause inhibition of pro-inflammatory cytokines, such as tissue necrosis factor-alpha (TNF-α) (38), and through vascular endothelial growth factor (VEGF) to stimulate angiogenesis (39). Finally, even Fe2+ with its binding to ferritin and the iron ATPase pump can have antioxidant, anti-inflammatory and anti-apoptotic effects (40). So wholesale inhibition of HO could have a myriad of potential adverse side effects while trying to modulate bilirubin production for the purpose of controlling TB levels during the transitional period after birth (41).
There are also a number of endogenous sources of CO (15). Heme degradation accounts for about 86% coming from senescing RBCs (~70%), ineffective erythropoiesis (~9%), and other hemoproteins (~21%) (42). The remaining 14% comes from lipid peroxidation (variable) and photo-oxidation (variable) (42). The latter sources can be quite large under pathologic conditions, such as lung injury or infection (43). Estimates of total bilirubin production by measuring CO excretion in breath, ETCOc, or COHbc levels have been performed in rodents and primates, including human infants (2,16). The validity of the method has been proven in a rat model of hemolysis in which a precise amount of heme can be recovered as CO in the breath of the animals. However, in most circumstances, certainly in clinical settings, the estimates are only approximations of the actual production rate of bilirubin because the exact contribution from non-heme sources cannot be known for certain without labeling techniques, which are not feasible in the clinical setting or without labeling of the carbon atom in the animal models (44,45). Nonetheless, estimates of total bilirubin production have been made for most clinical conditions in the human newborn. A variety of ETCOc devices have been used for estimating bilirubin production in babies (16).
With tools to estimate total bilirubin production, the ability to test drugs that could inhibit the process could be easily screened. The most important category of drugs for this purpose has been the heme analogs or metalloporphyrins (Mps), which are competitive inhibitors of HO (46-50). The criteria for selecting one of these compounds, most of which are synthetic, include the following desirable characteristics: contains a biocompatible central metal, potent HO inhibition, negligible degradation, negligible inhibition of other enzymes, negligible photoreactivity, optimal duration of action, and negligible HO upregulation (49). The HO-1-luciferase (-luc) transgenic mouse was created in order to monitor the effect of such inhibitors on HO-1 gene expression in living mice treated with the compounds by monitoring the emission of photons (bioluminescence) as HO-1 was expressed (51). This technique is useful for studying in vivo expression patterns and served originally as a model system for this technology because the HO reaction is tightly regulated due to the toxicity of CO, Fe2+, and bilirubin; tissue-specific expression; developmental regulation; its potential as a target for therapy; and the fact the ex vivo assays are slow and provide only a “snapshot” of the biological process (52). Thus, rapid screening for homozygosity is possible with bioluminescence imaging (BLI), and selected Mps could be easily screened for the effects on HO-1 gene expression (53). Although tin mesoporphyrin (SnMP) had many desirable characteristics, especially potency, and was introduced into clinical trials (54-57), it is photoreactive, had a protracted duration of action, and was not rapidly excreted or metabolized (58). Its approval for human use has been delayed for this reason (57,58). Zinc protoporphyrin (ZnPP), although less potent, is still effective, short-acting, and can be metabolized with release of an essential trace metal (49). Moreover, it is naturally occurring in humans (59). Studies in Rhesus monkeys (60) and newborn rats (61) with increased bilirubin production caused by hemolysis demonstrated its efficacy. Because it is difficult to keep in solution, a special formulation had to be prepared for its administration which could be delivered orally, giving it one more desirable characteristic (62,63). Compared to various possible formulations, a lipid preparation of ZnPP had adequate in vitro inhibitory potency and no chemical toxicity or phototoxicity (63).
Collectively, these studies have set the stage for further animal and ultimately human studies in order to ensure safety and efficacy. Notably, the ZnPP lipid formulation looks promising as an inhibitor of in vivo bilirubin production in a heme-loaded newborn mouse model (62). Combined with the identification of high bilirubin producers, such an approach would revolutionize the management of neonatal jaundice and provide a viable alternative to phototherapy where its risks might outweigh its benefits in the ELBW babies. Moreover, a new therapeutic paradigm might be possible. It would begin with identification and isolation of fetal cell-free (cf)RNA or cfDNA in the circulation of the mother (64,65), the creation of a genetic profile of jaundice risk looking for polymorphisms associated with decrease UGT1A1 activity (12), or HO-1 polymorphisms (66) followed by confirmation of any existing pathophysiology with noninvasive monitoring after birth, and targeting individual babies for chemoprevention, thus avoiding phototherapy altogether or at least minimizing its use. In this way, all the toxic effects of the HO/CO pathway might be mitigated while all the beneficial effects are retained.
Knowing whether a baby is a high producer of bilirubin is important for the clinician and should be available as a clinical measure, as it can be used to identify babies who would be likely to benefit from inhibition of bilirubin production. This approach would be disruptive to existing markets for phototherapy and change dramatically the management strategies for ELBW infants with hyperbilirubinemia.
Acknowledgments
Funding: This work was supported by the Charles B. and Ann L. Johnson Research Fund; the Christopher Hess Research Fund; the Providence Foundation Research Fund; the Roberts Foundation Research Fund; and the Stanford Maternal and Child Health Research Institute.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Pediatric Medicine for the series “Neonatal Jaundice”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm-21-8). The series “Neonatal Jaundice” was commissioned by the editorial office without any funding or sponsorship. DKS serves as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Pediatric Medicine from Oct 2020 to Sep 2022. RJW serves as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stevenson DK, Dennery PA, Hintz SR. Understanding newborn jaundice. J Perinatol 2001;21:S21-4; discussion S35-9. [Crossref] [PubMed]
- Wong RJ, Bhutani VK, Stevenson DK. The importance of hemolysis and its clinical detection in neonates with hyperbilirubinemia. Curr Pediatr Rev 2017;13:193-8. [Crossref] [PubMed]
- Stevenson DK, Vreman HJ, Wong RJ. Bilirubin production and the risk of bilirubin neurotoxicity. Semin Perinatol 2011;35:121-6. [PubMed]
- Johnson L, Bhutani VK. The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Perinatol 2011;35:101-13. [Crossref] [PubMed]
- Wong RJ, Stevenson DK. Neonatal hemolysis and risk of bilirubin-induced neurologic dysfunction. Semin Fetal Neonatal Med 2015;20:26-30. [Crossref] [PubMed]
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297-316. [PubMed]
- Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Engl J Med 2001;344:581-90. [Crossref] [PubMed]
- Stevenson DK, Wong RJ, Ostrander CR, et al. Increased carbon monoxide washout rates in newborn infants. Neonatology 2020;117:118-22. [Crossref] [PubMed]
- Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 1999;103:6-14. [Crossref] [PubMed]
- Dennery PA, McDonagh AF, Spitz DR, et al. Hyperbilirubinemia results in reduced oxidative injury in neonatal Gunn rats exposed to hyperoxia. Free Radic Biol Med 1995;19:395-404. [Crossref] [PubMed]
- Kaplan M, Renbaum P, Levy-Lahad E, et al. Gilbert syndrome and glucose-6-phosphate dehydrogenase deficiency: A dose-dependent genetic interaction crucial to neonatal hyperbilirubinemia. Proc Natl Acad Sci USA 1997;94:12128-32. [Crossref] [PubMed]
- Kaplan M, Hammerman C, Maisels MJ. Bilirubin genetics for the nongeneticist: hereditary defects of neonatal bilirubin conjugation. Pediatrics 2003;111:886-93. [Crossref] [PubMed]
- Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 1968;61:748-55. [Crossref] [PubMed]
- Vreman HJ, Mahoney JJ, Stevenson DK. Carbon monoxide and carboxyhemoglobin. Adv Pediatr 1995;42:303-34. [PubMed]
- Vreman HJ, Wong RJ, Stevenson DK. Carbon monoxide in breath, blood, and other tissues. In: Penney DG, editor. Carbon Monoxide Toxicity. Boca Raton: CRC Press; 2000:19-60.
- Tidmarsh GF, Wong RJ, Stevenson DK. End-tidal carbon monoxide and hemolysis. J Perinatol 2014;34:577-81. [Crossref] [PubMed]
- Ahlfors CE. Bilirubin-albumin binding and free bilirubin. J Perinatol 2001;21:S40-2; discussion S59-62. [Crossref] [PubMed]
- Lamola AA, Bhutani VK, Du L, et al. Neonatal bilirubin binding capacity discerns risk of neurological dysfunction. Pediatr Res 2015;77:334-9. [Crossref] [PubMed]
- Ahlfors CE, Bhutani VK, Wong RJ, et al. Bilirubin binding in jaundiced newborns: from bench to bedside? Pediatr Res 2018;84:494-8. [Crossref] [PubMed]
- Bhutani VK, Maisels MJ, Schutzman DL, et al. Identification of risk for neonatal haemolysis. Acta Paediatr 2018;107:1350-6. [Crossref] [PubMed]
- Bhutani VK, Srinivas S, Castillo Cuadrado ME, et al. Identification of neonatal haemolysis: an approach to predischarge management of neonatal hyperbilirubinemia. Acta Paediatr 2016;105:e189-94. [Crossref] [PubMed]
- Maisels MJ, Kring E. Rebound in serum bilirubin level following intensive phototherapy. Arch Pediatr Adolesc Med 2002;156:669-72. [Crossref] [PubMed]
- Cremer RJ, Perryman PW, Richards DH. Influence of light on the hyperbilirubinaemia of infants. Lancet 1958;1:1094-7. [Crossref] [PubMed]
- McDonagh AF, Lightner DA. Phototherapy and the photobiology of bilirubin. Semin Liver Dis 1988;8:272-83. [Crossref] [PubMed]
- Hansen TWR, Wong RJ, Stevenson DK. Molecular physiology and pathophysiology of bilirubin handling by the blood, liver, intestine, and brain in the newborn. Physiol Rev 2020;100:1291-346. [Crossref] [PubMed]
- Morris BH, Oh W, Tyson JE, et al. A multi-center randomized trial of aggressive versus conservative phototherapy for extremely low birth weight infants. N Engl J Med 2008;359:1885-96. [Crossref] [PubMed]
- Brown AK, Kim MH, Wu PY, et al. Efficacy of phototherapy in prevention and management of neonatal hyperbilirubinemia. Pediatrics 1985;75:393-400. [PubMed]
- Silverman WA. Ambitious overgeneralisation. Paediatr Perinat Epidemiol 2002;16:288-9. [Crossref] [PubMed]
- Arnold C, Tyson JE, Pedroza C, et al. Cycled phototherapy dose-finding study for extremely low-birth-weight infants: a randomized clinical trial. JAMA Pediatr 2020;174:649-56. [Crossref] [PubMed]
- Hintz SR, Stevenson DK, Yao Q, et al. Is phototherapy exposure associated with better or worse outcomes in 501- to 1000-g-birth-weight infants? Acta Paediatr 2011;100:960-5. [Crossref] [PubMed]
- Stevenson DK, Wong RJ, Arnold CC, et al. Phototherapy and the risk of photo-oxidative injury in extremely low birth weight infants. Clin Perinatol 2016;43:291-5. [Crossref] [PubMed]
- Stevenson DK, Rodgers PA, Vreman HJ. The use of metalloporphyrins for the chemoprevention of neonatal jaundice. Am J Dis Child 1989;143:353-6. [PubMed]
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 2008;60:79-127. [Crossref] [PubMed]
- Di Noia MA, Van Driesche S, Palmieri F, et al. Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome C oxidase activity in experimental diabetes. J Biol Chem 2006;281:15687-93. [Crossref] [PubMed]
- Calabrese V, Scapagnini G, Ravagna A, et al. Regional distribution of heme oxygenase, HSP70, and glutathione in brain: relevance for endogenous oxidant/antioxidant balance and stress tolerance. J Neurosci Res 2002;68:65-75. [Crossref] [PubMed]
- Sedlak TW, Snyder SH. Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics 2004;113:1776-82. [Crossref] [PubMed]
- Marks GS, Brien JF, Nakatsu K, et al. Does carbon monoxide have a physiological function? Trends Pharmacol Sci 1991;12:185-8. [Crossref] [PubMed]
- Brouard S, Berberat PO, Tobiasch E, et al. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem 2002;277:17950-61. [Crossref] [PubMed]
- Li Volti G, Sacerdoti D, Sangras B, et al. Carbon monoxide signaling in promoting angiogenesis in human microvessel endothelial cells. Antioxid Redox Signal 2005;7:704-10. [Crossref] [PubMed]
- Soares MP, Seldon MP, Gregoire IP, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol 2004;172:3553-63. [Crossref] [PubMed]
- Stevenson DK, Wong RJ. Metalloporphyrins in the management of neonatal hyperbilirubinemia. Semin Fetal Neonatal Med 2010;15:164-8. [Crossref] [PubMed]
- Vreman HJ, Wong RJ, Sanesi CA, et al. Simultaneous production of carbon monoxide and thiobarbituric acid reactive substances in rat tissue preparations by an iron-ascorbate system. Can J Physiol Pharmacol 1998;76:1057-65. [Crossref] [PubMed]
- van Bel F, Latour V, Vreman HJ, et al. Is carbon monoxide-mediated cyclic guanosine monophosphate production responsible for low blood pressure in neonatal respiratory distress syndrome? J Appl Physiol 1985;2005:1044-9. [PubMed]
- Johnson JD, Wetmore DL, Martinez CW, et al. Developmental changes in bilirubin production in the rat. J Pediatr Gastroenterol Nutr 1983;2:142-51. [Crossref] [PubMed]
- Salomon WL, Vreman HJ, Kwong LK, et al. Red cell destruction and bilirubin production in adult rats with short-term biliary obstruction. J Pediatr Gastroenterol Nutr 1986;5:806-10. [Crossref] [PubMed]
- Schulz S, Wong RJ, Vreman HJ, et al. Metalloporphyrins – an update. Front Pharmacol 2012;3:68. [Crossref] [PubMed]
- Vreman HJ, Cipkala DA, Stevenson DK. Characterization of porphyrin heme oxygenase inhibitors. Can J Physiol Pharmacol 1996;74:278-85. [PubMed]
- Vreman HJ, Ekstrand BC, Stevenson DK. Selection of metalloporphyrin heme oxygenase inhibitors based on potency and photoreactivity. Pediatr Res 1993;33:195-200. [Crossref] [PubMed]
- Vreman HJ, Wong RJ, Stevenson DK. Alternative metalloporphyrins for the treatment of neonatal jaundice. J Perinatol 2001;21:S108-13. [Crossref] [PubMed]
- Wong RJ, Vreman HJ, Schulz S, et al. In vitro inhibition of heme oxygenase isoenzymes by metalloporphyrins. J Perinatol 2011;31:S35-41. [Crossref] [PubMed]
- Zhang W, Feng JQ, Harris SE, et al. Rapid in vivo functional analysis of transgenes in mice using whole body imaging of luciferase expression. Transgenic Res 2001;10:423-34. [Crossref] [PubMed]
- Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng 2002;4:235-60. [Crossref] [PubMed]
- Zhang W, Contag PR, Hardy J, et al. Selection of potential therapeutics based on in vivo spatiotemporal transcription patterns of heme oxygenase-1. J Mol Med 2002;80:655-64. [Crossref] [PubMed]
- Kappas A, Drummond GS, Valaes T. A single dose of Sn-mesoporphyrin prevents development of severe hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient newborns. Pediatrics 2001;108:25-30. [Crossref] [PubMed]
- Martinez JC, Garcia HO, Otheguy LE, et al. Control of severe hyperbilirubinemia in full-term newborns with the inhibitor of bilirubin production Sn-mesoporphyrin. Pediatrics 1999;103:1-5. [Crossref] [PubMed]
- Valaes T, Petmezaki S, Henschke C, et al. Control of jaundice in preterm newborns by an inhibitor of bilirubin production: Studies with tin-mesoporphyrin. Pediatrics 1994;93:1-11. [PubMed]
- Bhutani VK, Poland R, Meloy LD, et al. Clinical trial of tin mesoporphyrin to prevent neonatal hyperbilirubinemia. J Perinatol 2016;36:533-9. [Crossref] [PubMed]
- Wong RJ, Bhutani VK, Vreman HJ, et al. Tin mesoporphyrin for the prevention of severe neonatal hyperbilirubinemia. NeoReviews 2007;8:e77-84. [Crossref]
- Labbé RF, Vreman HJ, Stevenson DK. Zinc Protoporphyrin: a metabolite with a mission. Clin Chem 1999;45:2060-72. [Crossref] [PubMed]
- Vreman HJ, Rodgers PA, Stevenson DK. Zinc protoporphyrin administration for suppression of increased bilirubin production by iatrogenic hemolysis in rhesus neonates. J Pediatr 1990;117:292-7. [Crossref] [PubMed]
- Rodgers PA, Seidman DS, Wei PL, et al. Duration of action and tissue distribution of zinc protoporphyrin in neonatal rats. Pediatr Res 1996;39:1041-9. [Crossref] [PubMed]
- Fujioka K, Kalish F, Wong RJ, et al. Inhibition of heme oxygenase activity using a microparticle formulation of zinc protoporphyrin in an acute hemolytic newborn mouse model. Pediatr Res 2016;79:251-7. [Crossref] [PubMed]
- Wong RJ, Schulz S, Espadas C, et al. Effects of light on metalloporphyrin-treated newborn mice. Acta Paediatr 2014;103:474-9. [Crossref] [PubMed]
- Koh W, Pan W, Gawad C, et al. Noninvasive in vivo monitoring of tissue-specific global gene expression in humans. Proc Natl Acad Sci U S A 2014;111:7361-6. [Crossref] [PubMed]
- Moufarrej MN, Wong RJ, Shaw GM, et al. Investigating pregnancy and its complications using circulating cell-free RNA in women's blood during gestation. Front Pediatr 2020;8:605219. [Crossref] [PubMed]
- Kaplan M, Renbaum P, Hammerman C, et al. Heme oxygenase-1 promoter polymorphisms and neonatal jaundice. Neonatology 2014;106:323-9. [Crossref] [PubMed]
Cite this article as: Stevenson DK, Wong RJ. The biology of bilirubin production: overview of detection and inhibition. Pediatr Med 2021;4:16.

