
Review of diagnosis and treatment of neonatal cardiorespiratory events
Diagnosis and treatment of neonatal cardiorespiratory events
The fetal to neonatal transition is associated with abrupt transformations in lung function, pulmonary circulation and respiratory control. This is clearly most challenging in the very preterm infant in whom immature respiratory control is superimposed upon an immature lung (1). However, the late preterm and term infants are also not exempt from this challenge. In 2019 I reviewed the short- and longer-term consequences of immature respiratory control in preterm infants (2) for this journal, and this remains an area of intense interest, while beyond this review, they range from retinopathy of prematurity (ROP) intraventricular hemorrhage and bronchopulmonary dysplasia (BPD) to longer term disability and mortality (2). It is important to recognize that these are associations with resultant intermittent hypoxic episodes and not necessarily causal. In this review I now seek to address diagnostic challenges associated with such episodes and focus on therapeutic options, both in the preterm and term neonate.
Monitoring respiration
There is widespread agreement that immature respiratory control leading to apneic episodes is the precipitant for most cardiorespiratory episodes leading to monitor alarms in preterm infants (Figure 1). Unfortunately, respiratory impedance monitoring has significant limitations, hence, it needs to be typically associated with heart rate monitors to detect accompanying bradycardia. The latter is probably a reflex response to hypoxemia in the absence of lung inflation, although bradycardia may occur in the absence of measurable desaturation.
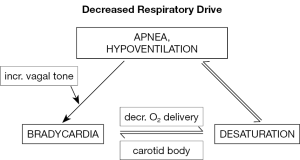
Impedance is widely used to non-invasively measure respiratory movements during hospitalization (3). Impedance monitoring has the advantage that it allows long-term measurements of respiration from electrocardiogram electrodes, although it is limited by its inability to distinguish normal respiration from obstructive efforts. This presents a considerable challenge as longer apneic episodes in preterm infants are most commonly mixed episodes comprising both central and obstructive components. Therefore, current bedside impedance monitors are limited to detection of central apnea.
A solution would be to measure airflow directly, or at least indirectly. For semi-qualitative measurements of flow, end-tidal CO2 or thermistor/thermocouples are alternative options. Devices placed on the face are, unfortunately, irritating to the infant. These devices have poor correlations with quantitative measures of flow and have, therefore, been limited to sleep lab settings where, when used in conjunction with chest wall motion sensors, can distinguish the presence or absence of flow occurring during central and obstructive apnea. Respiratory inductance plethysmography uses two bands placed around the rib cage and abdomen from which tidal volume can be inferred by the arithmetic sum of the displacements of these two compartments. Unfortunately, this methodology continues to be limited to short-term laboratory studies and has not found its way to routine clinical care settings (4).
Monitoring oxygenation
While arterial sampling is the “gold standard” for quantifying oxygenation, the high incidence of intermittent hypoxemia or desaturation events makes noninvasive continuous measurements imperative in most preterm infants. Transcutaneous PO2 was developed in the 1970s as the initial method of non-invasive monitoring. Unfortunately, measurements are adversely affected by increasing skin thickness with advancing postnatal maturation, peripheral hypoperfusion, and need for heating the electrode to at least 43 °C, as well as frequent calibrations. While the electrode response times are relatively slow at about 16 seconds, this methodology does have a place for ensuring levels of PaO2 are in range (5).
Given these reservations, pulse oximetry has become the most commonly used modality of non-invasive oxygenation monitoring in the NICU setting. The advantage of pulse oximetry is that it does not require heating of the skin, has a rapid response time, and is easy to apply. It can provide reliable values of oxygen saturation although the accuracy decreases with severe hypoxemia <70% owing to the inability to acquire physiological data for calibration curves beyond that range. Accuracy may also be affected by probe position, motion, light interference, low perfusion, skin pigmentation and variations in hemoglobin, the presence of fetal hemoglobin, and calibration algorithms.
Averaging times for pulse oximetry can be varied between the research and clinical settings. Longer averaging times may be preferred in the clinical setting to reduce the number of short desaturation events and false alarms. However, longer averaging times have been shown to falsely increase the number of prolonged events >20 s) (6). Although standards for both oxygen saturation targets and pulse oximeters vary somewhat between NICUs, this technique is a mainstay for detection of cardiorespiratory events and determining their potential consequences (7).
Providing ventilatory support
The definitive treatment for apnea/bradycardia/desaturation events during the NICU course of very preterm infants is intubation and mechanical ventilation. However, this is a step that we try to avoid where possible (Figure 2). Therefore, we typically resort to non-invasive ventilatory support. Continuous positive airway pressure (CPAP) has remained a mainstay of neonatal respiratory support, and has proven safe and effective since the 1970s (8). CPAP serves to maintain lung volume and enhance airway patency by means of low level positive pressure. This ensures patency of the upper airway during episodes of mixed and obstructive apnea. It, therefore, has a dual function to stabilize lung volume and improve airway patency by limiting upper airway closure. It is now clear that longer episodes of mixed apnea are compounded by pharyngeal or laryngeal upper airway obstruction. Thus, splinting of the upper airway by positive pressure becomes important. At the lower functional residual capacity characteristic of many preterm infants with residual lung disease, pulmonary oxygen stores are diminished, and the time from cessation of breathing to onset of desaturation and bradycardia can be quite short. Therefore, CPAP is likely to reduce this vulnerability to episodic desaturation. Although nasal CPAP is well tolerated in most preterm infants, low- or high-flow nasal cannula therapies are being increasingly used as an alternative and may allow CPAP delivery while enhancing mobility of the infant. Infants may benefit from the comfort of a nasal cannula over CPAP, but documentation of the pressure delivered is clearly comprised.
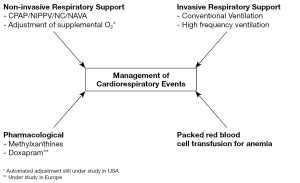
A non-invasive alternative is nasal intermittent positive pressure ventilation (NIPPV) which has been shown to decrease the risk of extubation failure (9). Such success may, however, depend on the ability to synchronize spontaneous and ventilator delivered breaths. Data from a smaller study that investigated a synchronized nasal ventilation system in 19 infants at a mean postmenstrual age of 30 weeks reported a decrease in bradycardia (<80 min-1) and desaturation rates (<80%) from 6.1 h-1 with CPAP to 2.9 h-1 with S-NIPPV (10). Unfortunately, synchronization of spontaneous breaths with ventilator delivered breaths remains a challenge. The alternatives include flow, pressure or neural stimuli from the infant to trigger the ventilator breath. Currently neurally assisted ventilatory support via an esophageal catheter seems to hold the most promise despite technical challenges (11).
Optimizing oxygenation
Intermittent hypoxemia or desaturation episodes are almost always the result of respiratory pauses, apnea, or ineffective ventilation (12). Lower oxygen saturation baseline predisposes to more frequent or profound intermittent hypoxemic episodes, although persistent baseline levels exceeding a 95% saturation should probably be avoided. Despite some controversy from earlier studies, administration of packed red blood cells (pRBC) in the face of anemia does decrease desaturation episodes. In infants with a hematocrit of 20–42%, pRBC transfusions were observed to decrease the incidence of intermittent hypoxia. This did not occur when infants only received a non-pRBC transfusion. This finding led to the obvious conclusion that the benefit of RBC transfusions is mediated through enhanced oxygen carrying capacity (13).
Automated control of inspired oxygen is an evolving approach whereby oxygenation saturation triggers delivery of supplemental oxygen (14). This automated technique has been compared to routine adjustments of inspired oxygen as performed by clinical personnel. During the automated period, time with oxygen saturation within the intended range increases significantly. This has been associated with modest reductions in both hyperoxemic and hypoxemic periods (15). It is important to acknowledge that if intermittent hypoxemia is the result of apnea, it may be more important to restore respiratory drive rather than just delivery of supplemental oxygen. Administration of excessive supplemental oxygen should be avoided when ventilatory support needs to be initiated because of its well-documented adverse effects.
Enhancing respiratory drive
During intrauterine life inspiratory efforts by the fetus may contribute to lung maturation, but they are not contributing to gas exchange. Such intermittent inspiratory efforts need to become continuous postnatally for an effective fetal to neonatal transition; this may not occur in the preterm infant. The primary therapeutic focus, in parallel with enhancing lung maturation and minimizing lung injury, is to enhance respiratory drive.
Caffeine is the “gold standard” and the most common therapy used to treat apnea and intermittent hypoxemia (16). Methylxanthines have been prescribed in preterm infants for over 40 years and have been shown to reduce apnea and the need for ventilation. The largest trial of caffeine (Caffeine for Apnea of Prematurity Trial) randomly assigned 2006 infants with birth weights between 500 and 1,250 gm to caffeine or placebo in the first 10 days of life. There was no attempt to measure apnea of prematurity in this study. However, therapy with caffeine reduced duration of positive-pressure support, oxygen supplementation, and the incidence of BPD (17). Survival without neurodevelopmental disability was also significantly improved at 18 to 21 months, and motor benefit was still noted at age 11 in the caffeine treated cohort (18).
Caffeine has a large variety of pharmacologic effects, although these are not well studied in the neonatal period. These include blockade of adenosine A1 and A2A receptor subtypes resulting in excitation of respiration neural output (19). A loading dose of caffeine showed a rapid (within 5 min) and prolonged (2 h) increase in diaphragmatic activity that was associated with an increase in tidal volume (20). Of particular interest, caffeine may exhibit anti-inflammatory properties directed toward the immature lung. For example, rat pups exposed prenatally to lipopolysaccharide had improved lung resistance and cytokine profiles after postnatal caffeine treatment (21).
Optimal strategies of caffeine therapy have yet to be determined. For example, common practice entails a caffeine citrate loading dose of 20 mg/kg followed by 5 to 10 mg/kg/day with potential side effects including tachycardia, dysrhythmia, feeding intolerance, gastroesophageal reflux disease, jitteriness, irritability, or rarely observed seizures. Excessive doses have been associated with an increased incidence of cerebellar hemorrhage (22). Therefore, only modest increases in dosing should be considered in selected patients.
There is a paucity of information from randomized control trials to address the optimal time to start or stop caffeine treatment. Such data are based on a few retrospective cohort studies. Early caffeine administration during the first 2 to 3 days of life was associated with reduction of BPD (23), patent ductus arteriosus requiring treatment, and duration of positive-pressure ventilation. This has resulted in widespread very early prophylactic administration of caffeine. American Academy of Pediatrics guidelines suggest discontinuing caffeine when cardiorespiratory events are insignificant for 5 to 7 days or 33 to 34 weeks’ postmenstrual age, whichever comes first (24). However, it is important to realize that preterm infants born at very young gestational ages may continue to have apnea and intermittent hypoxemia events even beyond 33 to 34 weeks’ postmenstrual age and prolonged therapy is under investigation (25).
Doxapram is a therapy which is being studied intensively in Europe (26). It stimulates peripheral and central chemoreceptors that result in augmentation of breathing. Significant reductions in apnea have been shown with increasing doses of doxapram. However, higher doses have been accompanied by elevations in blood pressure; prolonged use in the neonatal period has been associated with an adverse outcome in earlier studies. Because of the possibility of adverse effects of doxapram, this therapy should have limited appeal pending ongoing efficacy/safety studies.
Finally, non-pharmacologic approaches to enhancing respiratory drive have appeal because adverse effects are unlikely, even though efficacy appears limited. Such approaches have included kinesthetic stimulation using oscillating mattresses for prevention of apnea. In a meta-analysis that included 154 infants there was no clear evidence of effect of this therapy on apnea or bradycardia (27). By contrast, in a more recent study of preterm infants, stochastic (random) stimulation reduced desaturation episodes (28). There may well be a future for such novel modes of kinesthetic or related stimulation to improve respiratory drive.
Providing less evidence-based therapies
A variety to less evidence-based approaches have been attempted to decrease the frequency of cardiorespiratory events, especially as preterm infants approach discharge. These include enhancement of sensory stimulation as with skin-to-skin contact and music therapy (29). While beneficial data are very limited, preterm infants should be exposed to voices of family members, which includes reading, and the ambient noise levels in the NICU should be controlled so the infant can hear and discriminate human voices (30).
Apnea of prematurity with resultant desaturation and gastroesophageal reflux are both causally related. It is true that if refluxate reaches as high as the larynx or pharynx, an apneic response might result. Although this is theoretically possible, our data indicate that only about 3% of cardiorespiratory events (comprising apnea, bradycardia, and desaturation) are preceded by reflux (31). Furthermore, it is possible that concurrent reflux and apnea/desaturation are triggered by the hypoxic event, in which case reflux should not be the primary focus of therapy.
Inter-institutional and inter-provider variability in discharge monitoring is being increasingly addressed. A large study evaluated the effect of implementation of an institutional protocol for very low birth weight infants to reduce such inter-provider variability (32). This protocol determined standard definition of cardiorespiratory events and duration of monitoring in response to cardiorespiratory events. The result of the standardized protocol was not effective on length of stay, however, demonstrated a significant reduction in re-admission rate for apnea-related events. Similar protocols, when instituted as part of a quality improvement methodology, replicated improvements in reduction of variability, and found benefits to provider, nursing, and family satisfaction (33). With these measures we seek to avoid excessive duration of hospitalization without compromising safety. Fortunately, the use of home cardiorespiratory monitoring is discouraged and has declined over several decades as it can be highly disruptive in the home and provides little predictive data to guarantee infant safety.
Diagnosing and managing term infants with apnea
At or near term an infant should be developmentally and physiologically prepared for life beyond birth, given a more mature central respiratory control network and adequate airway size and lung development. Nonetheless, infants may require a NICU admission after an apneic or cyanotic episode is observed in the first days of life, often by a parent during an early feed (34). Unlike apnea of prematurity, which is well studied, there is much less information in the term or near term infant as this phenomenon is less common. Therefore, clear diagnostic criteria are inadequate with great variability in diagnostic testing and therapeutic approach. It should be noted that apnea in this population may well be an initial manifestation of a seizure.
Apnea in the delivery room may be the result of brain injury from hypoxia and ischemia (when associated with hypotension, hypoxemia, and metabolic acidosis), intrapartum maternal drug (e.g., narcotic or magnesium) administration, or general anesthesia and early onset sepsis. Other conditions should be considered in the early neonatal period, including neonatal stroke, central nervous system malformations, or metabolic causes (abnormalities in glucose, electrolytes, calcium). Sepsis always remains a potential concern. Poets et al. investigated the incidence of unexplained and sudden unexpected infant death in the first 24 hours of life in pediatric departments in German hospitals. When infants were placed in a potentially asphyxiating position this was identified as the significant risk factor (35).
It is imperative to obtain a thorough history and perform a detailed examination on any term infant presenting with an apneic or cyanotic episode. A careful review of the maternal, prenatal, intrapartum, resuscitation, and postpartum histories may provide important insight. This should include a detailed feeding history in all such infants. The extent of laboratory studies may vary, but should include the following: glucose, electrolytes, calcium, complete blood count, and blood gas determinations. Observation with cardiorespiratory and pulse oximetry monitoring in a NICU is usually recommended. More extensive investigation, such as neuroimaging, electroencephalogram, Otololaryngology consultation, and a Genetics evaluation should be considered based on the infant’s history, clinical course, and physical findings.
Gastroesophageal reflux has often been implicated as a cause of apnea in infancy. However, there is rarely a temporal relationship and, when one exists, it may well be the apnea-induced hypoxemia that results in relaxation of the lower esophageal sphincter and resultant gastroesophageal reflux (31,36). Acid suppression therapy is rarely indicated as the refluxate is often non-acidic (37). Furthermore, there is an increased risk of sepsis after acid suppression therapy (38).
In summary, apnea in the term infant is an unusual occurrence that requires consideration of a number of etiologies depending on whether it is central or obstructive and the presentation. Clinical judgment will be needed to dictate the extent of a diagnostic work-up and need for extended observation.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm-20-95). RJM serves as an unpaid editorial board member of Pediatric Medicine from Sep 2020 to Aug 2022. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Martin RJ. The unfortunate tale of immature respiratory control superimposed on an immature lung. Pediatr Res 2018;84:153-4. [Crossref] [PubMed]
- Martin RJ. The short- and longer-term consequences of immature respiratory control in preterm infants. Pediatr Med 2019;2:24. Review. [Crossref]
- Di Fiore JM, Poets CF, Gauda E, et al. Cardiorespiratory events in preterm infants: etiology and monitoring technologies. J Perinatol 2016;36:165-71. [Crossref] [PubMed]
- Brooks LJ, Di Fiore JM, Martin RJ, et al. Assessment of tidal volume over time in preterm infants using respiratory inductance plethysmography. Pediatr Pulmonol 1997;23:429-33. [Crossref] [PubMed]
- Poets CF, Southall DP. Noninvasive monitoring of oxygenation in infants and children: Practical considerations and areas of concern. Pediatrics 1994;93:737-46. [PubMed]
- Vagedes J, Poets CF, Dietz K. Averaging time, desaturation level, duration and extent. Arch Dis Child Fetal Neonatal Ed 2013;98:F265-6. [Crossref] [PubMed]
- Di Fiore JM, Bloom JN, Orge F, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr 2010;157:69-73. [Crossref] [PubMed]
- Di Fiore JM, Poets CF, Gauda E, et al. Cardiorespiratory events in preterm infants: Interventions and consequences. J Perinatol 2016;36:251-8. Review. [Crossref] [PubMed]
- Lemyre B, Davis PG, De Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPQAP) for apnea of prematurity. Cochrane Database Syst Rev 2000;CD002272. [PubMed]
- Gizzi C, Montecchia F, Panetta V, et al. Is synchronised NIPPV more effective than NIPPV and NCPAP in treating apnoea of prematurity (AOP)? A randomised cross-over trial. Arch Dis Child Fetal Neonatal Ed 2015;100:F17-23. [Crossref] [PubMed]
- Tabacaru CR, Moores RR Jr, Khoury J, et al. NAVA-synchronized compared to nonsynchronized noninvasive ventilation for apnea, bradycardia, and desaturation events in VLBW infants. Pediatr Pulmonol 2019;54:1742-6. [Crossref] [PubMed]
- Stryker CC, Dylag A, Martin RJ. Apnea and control of breathing. In: Jobe AH, Whitsett JA, Abman SH (eds): Fetal and Neonatal Lung Development: Clinical Correlates and Technologies for the Future. New York: Cambridge University Press, 2016.
- Kovatis KZ, Di Fiore JM, Martin RJ, et al. Effect of blood transfusions on intermittent hypoxic episodes in a prospective study of very low birth weight infants. J Pediatr 2020;222:65-70. [Crossref] [PubMed]
- Claure N, Bancalari E, D'Ugard C, et al. Multicenter crossover study of automated control of inspired oxygen in ventilated preterm infants. Pediatrics 2011;127:e76-83. [Crossref] [PubMed]
- van Kaam AH, Hummler HD, Wilinska M, et al. Automated versus manual oxygen control with different saturation targets and modes of respiratory support in preterm infants. J Pediatr 2015;167:545-50.e1-2.
- Shah VP, Di Fiore JM, Martin RJ: Respiratory control and apnea in premature infants. In: Bancalari E, Polin RA. editors. The Newborn Lung, 3rd ed. Philadelphia: Elsevier, 2018.
- Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med 2006;354:2112-21. [Crossref] [PubMed]
- Schmidt B, Roberts RS, Anderson PJ, et al. Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: An 11-year follow-up of the CAP Randomized Clinical Trial. JAMA Pediatr 2017;171:564-72. [Crossref] [PubMed]
- Wilson CG, Martin RJ, Jaber M, et al. Adenosine A2A receptors interact with GABAergic pathways to modulate respiration in neonatal piglets. Respir Physiol Neurobiol 2004;141:201-11. [Crossref] [PubMed]
- Kraaijenga JV, Hutten GJ, de Jongh FH, et al. The effect of caffeine on diaphragmatic activity and tidal volume in preterm infants. J Pediatr 2015;167:70-5. [Crossref] [PubMed]
- Köroğlu OA, MacFarlane PM, Balan KV, et al. Anti-inflammatory effect of caffeine is associated with improved lung function after lipopolysaccharide-induced amnionitis. Neonatology 2014;106:235-40. [Crossref] [PubMed]
- McPherson C, Neil JJ, Tjoeng TH, et al. A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr Res 2015;78:198-204. [Crossref] [PubMed]
- Lodha A, Seshia M, McMillan DD. Canadian Neonatal Network: Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA Pediatr 2015;169:33-8. [Crossref] [PubMed]
- Eichenwald ECCommittee on Fetus and Newborn, American Academy of Pediatrics. Apnea of Prematurity. Pediatrics 2016; [Crossref] [PubMed]
- Dobson NR, Patel RM, Smith PB, et al. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J Pediatr 2014;164:992-8.e3. [Crossref] [PubMed]
- Vliegenthart RJS, ten Hove CH, Onland W, et al. Doxapram treatment for apnea of prematurity: A systematic review. Neonatology 2017;111:162-71. [Crossref] [PubMed]
- Osborn DA, Henderson-Smart DJ. Kinesthetic stimulation versus theophylline for apnea in preterm infants. Cochrane Database Syst Rev 2000;1998:CD000502. [PubMed]
- Bloch-Salisbury E, Indic P, Bednarek F, et al. Stabilizing immature breathing patterns of preterm infants using stochastic mechanosensory stimulation. J Appl Physiol 2009;107:1017-27. [Crossref] [PubMed]
- Teckenberg-Jansson P, Huotilainen M, Pölkki T, et al. Rapid effects of neonatal music therapy combined with kangaroo care on prematurely-born infants. Nord J Music Ther 2011;20:22-42. [Crossref]
- Graven SN. Sound and the developing infant in the NICU: Conclusions and recommendations for care. J Perinatol 2000;20:S88-93. [Crossref] [PubMed]
- Di Fiore J, Arko M, Herynk B, et al. Characterization of cardiorespiratory events following gastroesophageal reflux in preterm infants. J Perinatol 2010;30:683-7. [Crossref] [PubMed]
- Chandrasekharan P, Rawat M, Reynolds AM, et al. Apnea, bradycardia and desaturation spells in premature infants: Impact of a protocol for the duration of 'spell-free' observation on interprovider variability and readmission rates. J Perinatol 2018;38:86-91. [Crossref] [PubMed]
- Coughlin K, Posencheg M, Orfe L, et al. Reducing variation in the management of apnea of prematurity in the intensive care nursery. Pediatrics 2020;145:e20190861. [Crossref] [PubMed]
- Patrinos ME, Martin RJ. Apnea in the term infant. Semin Fetal Neonatal Med 2017;22:240-4. Review. [Crossref] [PubMed]
- Poets CF, Bacher M. Treatment of upper airway obstruction and feeding problems in Robin-like phenotype. J Pediatr 2011;159:887-92. Review. [Crossref] [PubMed]
- Omari TI, Haslam RR, Lundborg P, et al. Effect of omeprazole on acid gastroesophageal reflux and gastric acidity in preterm infants with pathological acid reflux. J Pediatr Gastroenterol Nutr 2007;44:41-4. [Crossref] [PubMed]
- Corvaglia L, Zama D, Spizzichino M, et al. The frequency of apneas in very preterm infants is increased after non-acid gastro-esophageal reflux. Neurogastroenterol Motil 2011;23:303-7. [Crossref] [PubMed]
- Manzoni P, García Sánchez R, Meyer M, et al. Exposure to gastric acid inhibitors increases the risk of infection in preterm very low birth weight infants but concomitant administration of lactoferrin counteracts this effect. J Pediatr 2018;193:62-7.e1. [Crossref] [PubMed]
Cite this article as: Martin RJ. Review of diagnosis and treatment of neonatal cardiorespiratory events. Pediatr Med 2021;4:20.

