Kawasaki disease is not linked to COVID-19 in Chinese pediatric population
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) has become a global health emergency. Children infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have a milder disease course than infected adults (1). However, a cluster of children, who were tested positive for SARS-CoV-2 either by nasopharyngeal reverse transcription-polymerase chain reaction (RT-PCR) or serologic antibody testing, presented with hyperinflammatory syndrome similar to Kawasaki disease (KD) and toxic shock syndrome. This condition, provisionally termed pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 infection (PIMS-TS) or multisystem inflammatory syndrome in children (MIS-C), has been reported in the United Kingdom, Italy, France, and the United States (2-5). According to the definition from U.S. Centers for Disease Control and Prevention, MIS-C are diagnosed based on the following criteria: (I) an individual <21 years old presenting with fever, laboratory evidence of inflammation, and multisystem (>2) organ involvement (cardiac, kidney, respiratory, hematologic, gastrointestinal, dermatologic, or neurological); (II) no alternative plausible diagnoses; (III) positive for current or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or COVID-19 exposure within the 4 weeks prior to the onset of symptoms (6). According to the reports of Verdoni et al., Toubiana et al., and Whittaker et al., the common symptoms of MIS-C are incomplete KD, KD shock syndrome, macrophage activation syndrome (MAS), and gastrointestinal symptoms (2-4).
KD is a systemic vasculitis predominantly occurring in children under 5 years old. The etiology remains unknown and viral infection is considered to be one of the causes. In the Italian epicentre, Verdoni et al. reported a 30-fold higher incidence of Kawasaki-like disease during COVID-19 pandemic than that in the past 5 years (3). Similarly, in the French epicentre, Ouldali et al. identified a rapid increase of KD cases approximately 2 weeks after the peak of the COVID-19 epidemic (7), raising the possibility of SARS-CoV-2 being a trigger of KD. However, no dramatic increase in KD incidence was observed in Japan during the local epidemic (8). These conflicting results indicated that the relationship between SARS-Cov-2 infection and KD deserves further study. So far, this relationship has not been studied in Chinese population. Therefore, we conducted a multi-centre retrospective survey to investigate whether KD is linked to COVID-19 in Chinese pediatric population.
We present the following article/case in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/pm-20-112).
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Children’s Hospital of Fudan University (No. 2020442) and individual consent for this retrospective analysis was waived.
Study design and patient recruitment
This was a retrospective survey based on the 40 hospitals of China Kawasaki Disease Research Collaborative Group. Patients diagnosed with KD in these hospitals from January 1st, 2020 to April 30th, 2020 (corresponding to the COVID-19 epidemic period in China) were enrolled. KD was diagnosed according to the guidelines released by the American Heart Association in 2017 (9). Diagnostic criteria for complete KD were the presence of fever for at least 5 days together with at least four of the five following principle clinical features: (I) bilateral conjunctival injection; (II) polymorphous exanthema; (III) erythema and cracking of lips, strawberry tongue, and/or erythema of oral and pharyngeal mucosa; (IV) erythema and edema of the hands and feet in acute phase and/or periungual desquamation in subacute phase; (V) cervical lymphadenopathy (≥1.5 cm diameter). Incomplete KD, referring to KD patients with persistent fever and fewer than four principle symptoms, was diagnosed according to the algorithm in AHA guidelines. Patients admitted only for further evaluation and treatment of cardiac sequelae were excluded.
Data collection
Questionnaires were sent to the 40 hospitals. Clinical data were obtained from hospital medical records by pediatricians and further verified by a senior pediatric cardiologist in each hospital. For each patient, we recorded demographic information, diagnosis of KD shock syndrome and MAS, contact with confirmed or suspected cases of COVID-19, and results of RT-PCR tests and serologic antibody tests (IgM and IgG) for SARS-CoV-2. For each hospital, we recorded the number of KD cases diagnosed in January, February, March and April from 2017 to 2019 for comparison with the corresponding period in 2020.
Definitions
KD shock syndrome was defined on the basis of systolic hypotension for age, a sustained decrease in systolic blood pressure from baseline of ≥20%, or clinical signs of poor perfusion (10). MAS was defined according to the 2016 classification criteria for MAS in systemic juvenile idiopathic arthritis (11). Patients were classified as have MAS in the presence of ferritin >684 ng/mL and any two of the following criteria: (I) platelet count ≤181×109/L; (II) triglycerides >156 mg/dL; (III) fibrinogen ≤360 mg/dL; (IV) aspartate aminotransferase >48 units/L. Patients were considered to have current or recent SARS-CoV-2 infection if RT-PCR or antibody testing results were positive on admission for KD.
Statistical analysis
Categorical variables were presented as numbers and percentages, and continuous variables as mean (SD) or median (IQR). Generalized linear mixed models were performed to regress the numbers of KD cases by hospital and month (January to April) based on data from 2017 to 2019, treating hospital as fixed effect and year as random effect. The numbers of KD cases and 95% confidence intervals (95% CIs) were estimated for each hospital at overall level and by month, and 95% CIs were further used for hypothesis testing in comparison with the corresponding observed numbers in 2020. All missing data were reported. Analyses were performed using R software (version 4.0.0).
Results
Patient characteristics
Completed responses to the survey were received from 29 out of the 40 hospitals (72.5%) across 19 provinces in China (Figure 1). A total of 2,108 KD cases were reported from January to April 2020, including 1,345 male (63.8%) and 763 female (36.2%) cases. Age of onset ranged from 1 month to 13.8 years (median: 1.9 years). Complete and incomplete KD symptoms were observed in 1,775 (84.2%) and 333 (15.8%) cases, respectively.
KD shock syndrome and MAS
KD shock syndrome was diagnosed in eight patients (0.4%), including five males and three females (Table 1). Age of onset ranged from 2.0 to 7.5 years (median: 4.4 years). These patients came from different regions, two in Jilin, one in Sichuan, one in Henan, two in Chongqing, one in Gansu, and one in Guangdong. MAS was diagnosed in two male patients (0.1%), with one from Hubei and another from Liaoning (Table 1). Age of onset was 1.3 and 3.7 years, respectively. Incomplete KD symptoms were observed in one case of KD shock syndrome and one case of MAS.

Full table
Number of KD cases
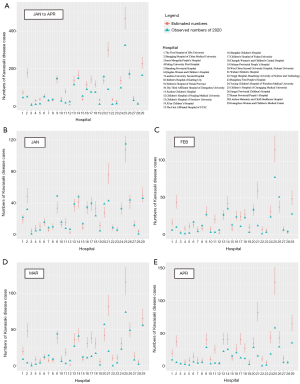
In overall, greater number of KD cases from January to April 2020 than the upper limit of 95% CI of estimated numbers of cases generated from the past 3 years were observed in only 2 (6.9%) out of 29 hospitals (Figure 2A). The stratified analyses by month showed that the number of hospitals with numbers of KD cases in 2020 exceeding the estimated numbers for the past 3 years were 3 (10.3%) in January, 3 (10.3%) in February, 3 (10.3%) in March, and none in April, respectively (Figure 2B,C,D,E).
Evidence of SARS-CoV-2 infection
Of all 2,108 patients, 434 (20.6%) received RT-PCR tests and 64 (3.0%) received serologic antibody tests for SARS-CoV-2 on admission for KD, all showing negative results, including one child diagnosed of COVID-19 35 days prior to KD onset and eight children who had likely contact history with COVID-19 patients. For the ten severe cases (with KD shock syndrome or MAS), none had contact history with COVID-19 patients (Table 1). RT-PCR was performed in two of the ten severe patients and also showed negative results.
Discussion
This study involved 29 hospitals from 19 provinces across China, reflecting the clinical features of KD in China during the COVID-19 pandemic. However, we did not find a link of KD with COVID-19 in Chinese pediatric population. While KD shock syndrome and MAS are rare severe complications of KD, they were diagnosed in over 50% of children with MIS-C in Western countries (2,3). Our data showed that the severe KD cases in China were much less common during the pandemic. KD shock syndrome (0.4%) in this study was less than that reported (1.5%) in Taiwan in 2013 (12) and MAS (0.1%) was less than that reported (1.1%) in Zhejiang province in 2015 (13). In addition, there are some differences in clinical features between severe KD patients and MIS-C children. Firstly, although it is difficult to distinguish MIS-C from KD shock syndrome due to the similar features of KD symptoms and hypotension, children with MIS-C were generally older than those with KD or with KD shock syndrome (median age: 9 vs. 2.7 vs. 3.8 years) (2). The median ages of children with KD and with KD shock syndrome in our study were 1.9 and 4.4 years, respectively, suggesting a difference between these children and those with MIS-C. Secondly, only two out of the ten severe KD patients had incomplete KD, while the proportion of incomplete KD in MIS-C children was nearly 50% (3,4). Thirdly, cases of KD shock syndrome or MAS were distributed in different provinces (one or two cases in each province), rather than concentrated in areas with higher rates of SARS-CoV-2 infection.
It is worth noting that MIS-C is currently considered as a post-infectious disease, which means that children’s antibodies against SARS-CoV-2 may produce an immune response in the body, and then produce symptoms similar to Kawasaki disease. The peak of MIS-C cases is observed approximately 2 to 4 weeks after the peak of COVID-19 cases (7,14). The number of COVID-19 cases in China emerged in December 2019 and reached a peak in February 2020. However, as compared with the past 3 years, a surge of KD cases were not observed in most hospitals during January to April 2020, indicating the incidence of KD has not increased during the COVID-19 epidemic period. This finding was contrary to the Italian and French researches (3,7), but consistent with the results of Iio’s research in Japan (8). No case of MIS-C was reported in Japan and China. One possibility to explain the discrepancies is that Japanese and Chinese children are not susceptible to MIS-C, which were found more prevalent in children of African descent (4,5,15). However, epidemiologic studies have revealed that the incidence of KD is much higher in Asian than in other areas (16,17), suggesting that MIS-C and KD are not the same disease. Another explanation is that studies with the finding of a link of KD with COVID-19 are limited by single-centre nature and a small number of patients. Recently, Quiat et al. found that there were no differences in antiviral antibody profiles between KD and matched febrile control sera using high-throughput sequencing (18). Therefore, the relationship between serologic antibodies and KD symptoms remains elusive and further studies are warranted.
Tests for COVID-19 were undertaken in a relatively small proportion of KD children in our study, which was mainly due to the limited knowledge of MIS-C at that time. Therefore, a recent or current SARS-CoV-2 infection could neither be confirmed nor excluded in most children at the onset of KD. However, no increase was found in the number of KD cases and the proportion of severe cases during the COVID-19 epidemic, which did not indicate an association between COVID-19 and KD. In addition, we found there were some differences in clinical features between ten severe KD patients in our study and reported MIS-C children in terms of age of onset, the proportion of incomplete KD, and geographical distribution. Furthermore, only 9 of 2,108 KD patients had exposure history of COVID-19 in our study. Those with exposure history of COVID-19 did not have severe disease courses, and those with severe disease courses did not have exposure history of COVID-19. All these indicate that their illness is not likely caused by SARS-CoV-2 infection, which is supported by the absence of KD symptoms in Chinese children with SARS-CoV-2 infection (1,19,20).
Except for SARS-CoV-2, there are six coronaviruses known to infect humans, including HCoV-NL63, HCoV-229E, HCoV-OC43, HCoV-HKU1, SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV). In 2005, Esper et al. found that KD children have a higher rate of HCoV-NL63 infection than those controls with nonspecific respiratory symptoms (8/11 vs. 1/22), indicating a possible association between KD and HCoV-NL63 (21). However, the association was not confirmed in the subsequent studies by Shimizu et al. (1/48 KD children tested positive for HCoV-NL63) (22), Ebihara et al. (0/19 KD children vs. 5/208 controls with respiratory tract disease tested positive) (23), and Chang et al. (all 53 KD children tested negative) (24). In 2014, Shirato et al. found that HCoV-229E was related to the development of KD as HCoV-229E showed a higher positivity in the sera from KD patients when compared with healthy controls (25). In 2016, Gira et al. reported that HCoV-OC43 and HCoV-HKU1 were detected on the upper respiratory tract specimen of a 17-month-old KD girl, indicating a possible association between KD and HCoV-OC43/HCoV-HKU1 infection (26). However, the association of three human coronaviruses (HCoV-229E, HCoV-OC43, and HCoV-HKU1) and KD has not been confirmed in other studies. There are no reports on the relationship between KD and other two novel coronaviruses (SARS-CoV-1 and MERS-CoV). In 2021, Choe’s study demonstrated no temporal association between human coronavirus and KD at the national level in South Korea (27). Given all these, it has not been confirmed that coronavirus can cause KD.
The retrospective design of our study has its inevitable limitations. However, currently, the retrospective research is the only feasible study design for our study. We applied the data of year 2020 and of the past 3 years rather than using even earlier data to minimize the uncertainties. Further prospective studies are needed to investigate the relationship between KD and COVID-19, for example, to test if the infection rate of SARS-CoV-2 in KD patients is significantly higher than that in other pediatric patients.
In conclusion, our current study did not provide evidence of the link of KD with COVID-19 in China in terms of its prevalence and severity. However, it may highlight the important role of different subtypes of SARS-CoV-2 and ethnic differences in genetic background in the disease course of COVID-19 patients. Since the association of COVID-19 and MIS-C has become new concerns all over the world, further study is warranted to clarify the potential link between KD with COVID-19 in children.
Acknowledgments
We would like to express our sincere gratitude to all of the hospitals and investigators of China Kawasaki Disease Research Collaborative Group that involved in this survey, including Fang Liu, MD, Liping Xie, MD, Yin Wang, MD, Weili Yan, PhD, Guoying Huang, MD, Yixiang Lin, MD, Lan He, MD, Yi Zhang, MD (Children’s Hospital of Fudan University, Shanghai, China); Xiaoyan Liu, MD, Qijian Yi, MD, Hongmei Xu, MD, Ruiqiu Zhao, MD, Enmei Liu, MD (Children’s Hospital of Chongqing Medical University, Chongqing, China); Xiaohui Liu, MD, Fang Xie, MD (Jiangxi Provincial Children’s Hospital, Nanchang, China); Ping Huang, MD, Wei Li, MD (Guangzhou Women Children’s Medical Center, Guangzhou, China); Xiaoqing Shi, MD, Juan Gong, MD (West China Second University Hospital, Sichuan University, Chengdu, China); Yingjun Feng, MD, Ruili Zheng, MD, Xiaoli Yao, MD (Children’s Hospital of Henan Province, Zhengzhou, China); Juanli Wang, MD, Hongyu Xiao, MD (Xi’an Children’s Hospital, Xi’an, China); Haitao Lv, MD, Ling Sun, MD, Ye Chen, MD (Children’s Hospital of Soochow University, Suzhou, China); Maoping Chu, MD, Rongzhou Wu, MD, Yuanhai Zhang, MD (Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China); Min Huang, MD, Lijian Xie, MD, Sirui Song, MD (Shanghai Children’s Hospital, Shanghai, China); Yong Zhang, MD, Yuan Long, MD (Wuhan Children’s Hospital, Wuhan, China); Yiling Liu, MD, Yanfeng Yang, MD, Yingzi Zhang, MD (Chengdu Women’s Children’s Central Hospital, Chengdu, China); Mingwu Chen, MD, Tao Fang, MD (The First Affiliated Hospital of University of Science Technology of China, Hefei, China); Ce Wang, MD, Yunming Xu, MD (Shengjing Hospital of China Medical University, Shenyang, China); Silin Pan, MD, Zipu Li, MD, Zhixian Ji, MD (Qingdao Women Children’s Hospital, Qingdao, China); Jinhua Piao, MD, Lianhua Jin, MD (the First Hospital of Jilin University, Changchun, China); Zhenyu Xiong, MD, Yuanyuan Zhu, MD (Children’s Hospital of Kaifeng City, Kaifeng, China); Xuehua He, MD, Yonghua Yuan, MD (Hunan Provincial People’s Hospital, Changsha, China); Xiangyu Dong, MD, Li Min, MD, Yinan Yang, MD (Lanzhou University Second Hospital, Lanzhou, China); Yan Li, MD, Yu Liang, MD, Jun Wang, MD (the Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China); Shiwei Yang, MD, Jie Yin, MD (Children’s Hospital of Nanjing Medical University, Nanjing, China); Xinjiang An, MD, Jing Tian, MD (Xuzhou Children’s Hospital, Xuzhou, China); Xiaoli Li, MD, Xianmei Huang, MD (Hangzhou First People’s Hospital, Hangzhou, China); Bo Han, MD, Yingchun Yi, MD (Shandong Provincial Hospital, Jinan, China); Yanling Liao, MD, Xiaoli Liu, MD (Liuzhou Maternity Child Healthcare Hospital, Liuzhou, China); Qian Peng, MD, Xiaoping Hu, MD, Bo Li, MD (Sichuan Provincial People’s Hospital, Chengdu, China); Hui Yan, MD, Wei Bai, MD (Peking University First Hospital, Beijing, China); Huiling Lu, MD, Xiufen Hu, MD, Xiaoping Luo, MD (Tongji Hospital Affiliated to Tongji Medical College, Wuhan, China); Hua Zhu, MD, Yanyan Liang, MD (Inner Mongolia People’s Hospital, Hohhot, China).
Funding: This work was supported by Research Units of Early Intervention of Genetically Related Childhood Cardiovascular Disease, Chinese Academy of Medical Sciences (2019-I2M-5-002); and Science and Technology Commission of Shanghai Municipality (20ZR1408500).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/pm-20-112
Data Sharing Statement: Available at http://dx.doi.org/10.21037/pm-20-112
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm-20-112). GH serves as the Editor-in-Chief of Pediatric Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Children’s Hospital of Fudan University (No. 2020442) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med 2020;382:1663-5. [Crossref] [PubMed]
- Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020;324:259-69. [Crossref] [PubMed]
- Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395:1771-8. [Crossref] [PubMed]
- Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020;369:m2094. [Crossref] [PubMed]
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020;383:334-46. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Emergency preparedness and response: health alert network. Published May 14, 2020. Accessed February 25, 2021. Available online: https://emergency.cdc.gov/han/2020/han00432.asp
- Ouldali N, Pouletty M, Mariani P, et al. Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health 2020;4:662-8. [Crossref] [PubMed]
- Iio K, Uda K, Hataya H, et al. Kawasaki disease or Kawasaki-like disease: influence of SARS-CoV-2 infections in Japan. Acta Paediatr 2021;110:600-1. [Crossref] [PubMed]
- McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017;135:e927-99. [Crossref] [PubMed]
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics 2009;123:e783-9. [Crossref] [PubMed]
- Ravelli A, Minoia F, Davì S, et al. 2016 Classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis 2016;75:481-9. [Crossref] [PubMed]
- Lin MT, Fu CM, Huang SK, et al. Population-based study of Kawasaki disease shock syndrome in Taiwan. Pediatr Infect Dis J 2013;32:1384-6. [Crossref] [PubMed]
- Wang W, Gong F, Zhu W, et al. Macrophage activation syndrome in Kawasaki disease: more common than we thought? Semin Arthritis Rheum 2015;44:405-10. [Crossref] [PubMed]
- Felsenstein S, Willis E, Lythgoe H, et al. Presentation, treatment response and short-term outcomes in paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 (PIMS-TS). J Clin Med 2020;9:3293. [Crossref] [PubMed]
- Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395:1607-8. [Crossref] [PubMed]
- Ae R, Makino N, Kosami K, et al. Epidemiology, treatments, and cardiac complications in patients with Kawasaki disease: the nationwide survey in Japan, 2017-2018. J Pediatr 2020;225:23-9.e2. [Crossref] [PubMed]
- Xie LP, Yan WL, Huang M, et al. Epidemiologic features of Kawasaki disease in Shanghai from 2013 through 2017. J Epidemiol 2020;30:429-35. [Crossref] [PubMed]
- Quiat D, Kula T, Shimizu C, et al. High-throughput screening of Kawasaki disease sera for antiviral antibodies. J Infect Dis 2020;222:1853-7. [Crossref] [PubMed]
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708-20. [Crossref] [PubMed]
- Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 2020;26:502-5. [Crossref] [PubMed]
- Esper F, Shapiro ED, Weibel C, et al. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis 2005;191:499-502. [Crossref] [PubMed]
- Shimizu C, Shike H, Baker SC, et al. Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease. J Infect Dis 2005;192:1767-71. [Crossref] [PubMed]
- Ebihara T, Endo R, Ma X, et al. Lack of association between New Haven coronavirus and Kawasaki disease. J Infect Dis 2005;192:351-2; author reply 353. [Crossref] [PubMed]
- Chang LY, Chiang BL, Kao CL, et al. Lack of association between infection with a novel human coronavirus (HCoV), HCoV-NH, and Kawasaki disease in Taiwan. J Infect Dis 2006;193:283-6. [Crossref] [PubMed]
- Shirato K, Imada Y, Kawase M, et al. Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J Med Virol 2014;86:2146-53. [Crossref] [PubMed]
- Giray T, Biçer S, Küçük Ö, et al. Four cases with Kawasaki disease and viral infection: aetiology or association. Infez Med 2016;24:340-4. [PubMed]
- Choe SA, An HS, Choe YJ. No temporal association between human coronavirus and Kawasaki disease: national data from South Korea. J Med Virol 2021;93:585-7. [Crossref] [PubMed]
Cite this article as: Liu F, Xie L, Wang Y, Yan W, Huang G; on behalf of the study team of China Kawasaki Disease Research Collaborative Group. Kawasaki disease is not linked to COVID-19 in Chinese pediatric population. Pediatr Med 2021;4:23.