
Bilirubin neurotoxicity: a narrative review on long lasting, insidious, and dangerous effects
Introduction
The features of bilirubin deposition in the brain were initially described by Orth (1) and later designated as ”kernicterus” by Schmorl (2) in the last quarter of the 19th century. Today, more than a century later, and despite the extensive research, multiple management recommendations and guidelines (3-8), cases of acute bilirubin encephalopathy (ABE) are still being described during the early neonatal period (9,10), particularly in low- and/or middle-income countries (11-14). Recent reviews recapitulate the spectrum of disorders associated with bilirubin neurotoxicity and kernicterus, highlighting the toxic role of elevated free bilirubin (Bf) levels (15), i.e., unconjugated bilirubin not bound to its main blood transporter, albumin. They also emphasize several risk factors and co-morbidities that can lead to increased concentrations of serum albumin-bound unconjugated bilirubin (UCB), accounting for elevated Bf levels and subsequent neurotoxicities (Figure 1). Moreover, the available preventive and treatment options, as well as the recommendations are identified to manage ABE and kernicterus spectrum disorders (KSD) (16-20).
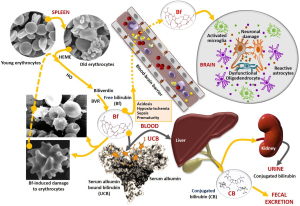
With regards to the neurotoxic actions of UCB, four main factors, acting alone or in combination, are implicated as illustrated in Figure 1: increased bilirubin production, impaired hepatic uptake, reduced bilirubin conjugation, and defective liver clearance (21). The excessive production of UCB in the first days of life primarily derives from the relative polycythemia and breakdown of hemoglobin, as well as from the increased red blood cell (RBC) turnover in neonates, with a rate of 6 to 8 mg/kg/day, more than twice the production as adults (22). Bilirubin is generated from heme degradation, catalyzed by heme-oxygenase (HO) to form biliverdin, which is then metabolized by biliverdin reductase (BVR) to Bf or UCB (if bound to albumin) (17). Other risk factors are implicated in UCB overproduction. This is the case for hemolytic diseases, e.g., glucose-6-phosphate dehydrogenase (G6PD) deficiency with increased erythrocyte fragility and hemolysis (23,24). The relative prevalence of G6PD deficiency (25), associated with neonatal hyperbilirubinemia (26) and prematurity (27), makes both conditions significant risk factors. Notably, UCB can bind to RBCs (28,29) causing shape alterations and increased fragility that culminate in increased hemolysis, further enhancing UCB and Bf production (Figure 1) (30-33).
UCB dissociates from albumin before entering the liver and may be impacted by decreased delivery or by inefficient hepatocyte uptake due to sinusoidal protein polymorphisms (21,34). Low hepatic gene expression of the bilirubin uridine diphospho-glucuronosyltransferase 1A1 (UGT1A1), as well as UGT1A1 enzyme deficiency in Gilbert’s disease (partial) and Crigler-Najjar types I (total, CN1) and II (almost total, CN2) syndromes, impairs bilirubin conjugation with (mostly) glucuronic acid (35-37), thus leading to increased levels of UCB and Bf in circulation. Enzyme polymorphisms may also play a role (35,38). Of note, CN1 syndrome leads to fatal outcomes with kernicteric features, unless liver transplantation is performed (39). Finally, excretion of conjugated bilirubin into the bile, mainly mediated by multidrug resistance-associated protein 2 (MRP2), is a key player for conjugated bilirubin elimination from the liver (40) and stool output (41). MRP2 deficiencies may cause the re-uptake of conjugated bilirubin into circulation (Dubin-Johnson syndrome) and the presence of cholestasis may lead to its elimination in urine (42).
Other main risk factors for bilirubin-induced neurological damage (BIND) during neonatal hyperbilirubinemia are: (I) prematurity that affects all the UCB clearance mechanisms and increase neural cell susceptibilities to its harmful effects (10,43-47); and (II) hypoxia-ischemia (48), sepsis (49), hypoalbuminemia (50) and acidosis (31,51,52) that contribute to increase Bf concentrations and its entrance in the central nervous system (CNS) after crossing the blood-brain barrier (BBB) (Figure 1), causing neuronal damage and glial activation.
Breastfeeding has also been associated with an increased incidence of hyperbilirubinemia, but the causes for “breast milk jaundice” or “breastfeeding failure jaundice” are not completely clear (53,54). However, an association with intestinal flora colonization status has been recently described (55,56). This may be important since it has been reported that the lack of microbiota in jaundiced babies may lead to the reabsorption of non-polar UCB in the intestine and may contribute to the development of BIND (57).
A less considered risk factor for UCB neurotoxicity in neonates is the apparent lack of societal awareness for this condition (58), together with early discharge policies practiced by birthing centers and maternity services that impair early detection and timely therapeutics, which are crucial to prevent UCB encephalopathies (59).
In summary, newborn infants overproduce UCB and have a decreased ability to eliminate UCB, thus increasing their susceptibility for UCB-induced neurodegeneration, oligodendrocyte dysfunction, astrocyte reactivity, and microglia activation in specific brain regions, which can lead to neurological sequelae with different severe long-term morbidities.
This review summarizes the concepts associated with UCB, Bf, and erythrocyte-linked neurotoxic species, using descriptive neuro- and gliocentric views, and addressing the key role of intercellular paracrine dysregulation to homeostatic imbalance and BIND. Future research using human advanced models and extracellular vesicles (EVs) to clarify pathological mechanisms associated with BIND and long-term sequelae are outlined, and the relevance of their use as new therapeutic tools in personalized medicine are also addressed.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-37/rc).
Methods
We conducted an exhaustive literature search on the electronic databases including PubMed, MEDLINE and Google Scholar, from January 1947 to April 2021, to identify all relevant studies mentioning serum bilirubin in relation to neonatal hyperbilirubinemia, bilirubin encephalopathy and kernicterus. A combination of the search terms included UCB-induced neurodegeneration, unbound or free bilirubin, bilirubin binding to albumin, bilirubin binding capacity of albumin, bilirubin-induced neurotoxicity, red cell binding of bilirubin, erythrocyte-bound bilirubin, neonatal jaundice, bilirubin metabolism in infants, bilirubin uptake, conjugation and clearance by the liver, bilirubin encephalopathy, kernicterus, oligodendrocytes, astrocytes, neurons and microglia. Languages other than English, German and French were excluded. Selection included clinical, animal and cellular studies. The selection process was conducted by both Authors, following agreement on search criteria, and selected references shared in a common database. The reference list also included studies identified manually, and studies referenced for other purposes.
Pathological implications of neurotoxic bilirubin species
Before addressing the neuropathological effects of the increased concentrations of UCB and Bf, it is perhaps worthwhile to describe the controversies regarding the beneficial and harmful effects of UCB, which directly depend on its “physiological” (slightly elevated) or markedly increased concentrations. The pleiotropic role of UCB at low levels as an antioxidant (60-62), though still controversial (63,64), and its anti-inflammatory effects (65-67), have contributed to a poor understanding, and sometimes even a dismissive attitude towards the harmful consequences of high UCB levels, either in neonatal life or as a consequence of inherited unconjugated hyperbilirubinemias, such as Gilbert, CN1 and CN2 syndromes. As an example, a shift between antioxidant and pro-oxidant actions may occur between intracellular UCB values of 7 ng/mg protein and those above 25 ng/mg protein, respectively (68). The dual effects of UCB (Figure 2) are even more difficult to understand when several therapeutic approaches have used low concentrations of UCB to treat several pathologies based on its antioxidant and anti-inflammatory properties (66,69-74). However, the harmful effects of UCB at high concentrations and the severe neurological consequences that unfortunately still occur should not be disregarded (75).
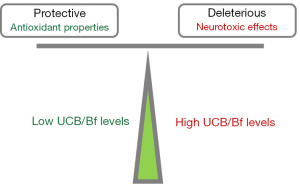
For that reason, we will focus on neuropathological issues, brain lesions and sequelae resulting from ABE and chronic kernicterus most often associated with bilirubin levels of 19 mg/dL or higher (16), which have been mostly addressed (53). In contrast, the life-long consequences of moderate levels of total or UCB, such as those surpassing 5 mg/dL in the first 2 to 4 days and up to values below 18 mg/dL (76,77) on the CNS are still unknown. One consequence is the association between neonatal hyperbilirubinemia and autism spectrum disorder (78), which has been only suspected before or even denied (53,79). Auditory brainstem function is also impaired in neonates with hyperbilirubinemia (80), and hearing screening tests have shown a relevant association between bilirubin levels and abnormal auditory activity in jaundiced newborns (81) that have been associated with KSD (5,16,82,83). As would be expected, the risk for auditory damage is increased in preterm infants, where bilirubin levels considered “safe” for term babies can lead to irreversible lesions (84). Alterations of the sensorimotor system due to elevated UCB concentrations assessed at several developmental ages have been reviewed by Lunsing (85). Abnormalities in the visuocortical function were observed at 3 months of age in children who had total bilirubin levels between 10 and 25 mg/dL at postnatal (PN) day 3 (86). Delayed neurodevelopmental outcomes at 6 months (87) and 1 year of age (88) were also found in term newborns, even with moderate hyperbilirubinemia accordingly to recent prospective cohort studies performed in India.
Though usually not associated with cognitive abnormalities, the literature is divided on this issue (89-93). Neurobehavioral disabilities with lower rates of school completion and full-time employment, as well as reading difficulties, were related to the existence of hyperbilirubinemia (94). Interestingly, a recent study using hippocampal neurons and animal models revealed that UCB induces the deposition of the amyloid-β (Aβ) peptide and tau hyperphosphorylation, establishing a link between an early exposure to bilirubin and Alzheimer’s disease (AD) features in later life (95), thus reinforcing its long-term effects. In a later study, high UCB levels, together with decreased serum concentrations of albumin, were found in dementia patients with Aβ and intravenous administration of albumin produced beneficial effects on daily function and dementia severity in AD patients (96).
In conclusion, neonatal-associated UCB and BIND may contribute to auditory and motor deficits (97), but also be associated with developmental delay, cognitive impairment, behavioral problems as well as poor executive function, and psychiatric disorders (98,99).
Free and erythrocyte-bound bilirubin
Bf was first designated as the fraction of bilirubin that was not conjugated and bound to albumin, distinct from the conjugated species. This concept was introduced in 1958 with an Italian publication (100), followed by a French one (101) (and many others) until 1969 to 1972, when the low concentration of the non-protein-bound bilirubin species started to be estimated and was determined to be around 10−10 or 10−9 mol/L (102,103). The authors, at that time, designated this fraction as Bf or unbound and suggested that it could increase under some conditions to 10−6 or 10−5 mol/L. First determinations used the Sephadex G-25 elution technique for the separation of Bf and albumin-bound bilirubin (104,105) and the enzymatic oxidation with hydrogen peroxide and horseradish peroxidase (106). The former method was even commercialized and recognized as a valuable aid to neonatologists in preventing bilirubin encephalopathy (107,108). Then, when the two processes were compared, the peroxidase method was found to require less volume of serum and to be more sensitive for the assessment of Bf concentration (109). All these studies were fundamental to finally separate two species of UCB, the one bound to albumin and the free species, which is the most toxic fraction (15,110,111). Determination of Bf and the estimation of reserve albumin binding capacity was then complemented by the erythrocyte-bound bilirubin (29,112). All these methods were thereafter reviewed (113). Now, some studies have assessed the modifications caused by the binding of bilirubin to erythrocytes, either for morphological changes or induced hemolysis, and consequences that it could have in aggravating the risk of BIND (Figure 1) (30,114). Another important contribution to the relevance of the toxic levels of Bf was the understanding about the bilirubin displacement from albumin by competitive binding of endogenous compounds and several drugs that promoted an increase of its levels (106,115-118).
Neurocentric view of BIND
The notion that UCB reaches the brain by crossing the BBB was probably concluded from studies performed in the mid-1960s using various animal models of experimental bilirubin encephalopathy (119). Such studies also suggested that neurologic damage was related to UCB concentration in the brain. Furthermore, UCB seemed not to be a passive player in BBB dynamic properties, but it possibly could trigger several damaging mechanisms that impair the barrier function at the level of brain microvascular endothelial cells (120) in a time-dependent manner (121). These effects were observed both in vitro and in post-mortem brain sections of infants with kernicterus (122).
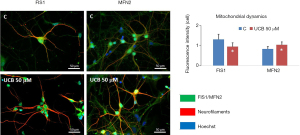
Once in the brain, UCB interacts with neurons and may cause irreversible damage. Initial studies in experimental kernicterus already proposed that UCB diffuses through the neuroplasm, interacting with the Golgi complex, neurotubules, and endoplasmic reticulum (ER) of neurons, diffusing into the axoplasm and causing axonal destruction (123). Another long-recognized target for UCB is the mitochondria, where UCB damages respiration, uncouples oxidative phosphorylation, and induces brain mitochondrial swelling, even at low micromolar concentrations (124,125). We found that UCB also impairs the mechanisms associated with mitochondrial fusion-fission dynamics as depicted in Figure 3 (unpublished data), which are associated with the maintenance of cellular quality (126). Elevated mitochondrial fusion, here assessed by mitofusin 2 protein immunostaining, favors the generation of interconnected mitochondria to increase cell bioenergetics efficiency when facing an insult as a cell survival mechanism (127). In contrast, fission that we determined through the expression of the mitochondrial fission 1 protein (FIS1) is associated with numerous mitochondrial fragments and its decrease may lead to reduced mitochondria motility (128). UCB also triggers mitochondrial membrane permeabilization, with the release of cytochrome c, and activation of caspases 3 and 9, that culminate in neuronal apoptosis as described previously (129,130) and us as well (131).
Although the exact toxic mechanisms are still not clear, it is becoming apparent that UCB impairs neuronal cells by a plethora of effects that eventually culminate in cell death by necrosis- and apoptosis-like mechanisms (132,133), which may involve glutamate excitotoxicity (134). In fact, several reviews describe multiple neurotoxic mechanisms for UCB, like inhibition of neurite outgrowth and ramification (135), alteration of neuronal membrane microfluidity, impairment of axonal arborization, and increased nitrosative stress (136) that, together with glutamate, seem to mediate arborization impairment (137) and to alter synaptic transmission (138). Not surprisingly, immature cells appear to be more sensitive to UCB neurotoxicity (139,140), correlating with the proposed age-related window of susceptibility to UCB neurological damage (141). It is important to note that most of these results were obtained in experimental conditions that mimic the true pathophysiological conditions, i.e., with clinically relevant molar ratios of UCB compared with human serum albumin (HSA), avoiding excessive aggregation and precipitation (142).
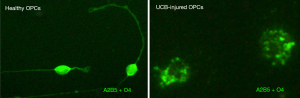
Studies using live calcium imaging reinforce the role of ER stress in UCB neurotoxicity to hippocampal neurons previously described as one of the most UCB-susceptible neuronal subpopulations (143,144), with a disruption of calcium homeostasis in neuronal cells, but not in astrocytes. This agrees with the previous report of Qaisiya et al. showing the involvement of ER stress in neuroinflammation and apoptosis in the SH-SY5Y differentiated neuronal cells (145). Interestingly, a similar mechanism involving ER stress and calpain (an intracellular Ca2+-dependent cysteine protease) was shown to interfere with oligodendrocyte maturation by UCB-induced demise of oligodendrocyte precursor cells (Figure 4) (146), impacting axonal myelination (147) and thus, potentially disturbing axonal conduction and, consequently, neuronal communication.
Other novel findings highlight the potential of UCB to disrupt neuronal communication, such as the inhibition of lipid raft-dependent functions at the specific level of the nerve cell adhesion molecule 1 (L1) that is involved in neuronal signaling (148). Using patch-clamp techniques, Shi et al. found that UCB increased the spontaneous firing rates of neonatal neurons in brainstem slices in a calcium-dependent manner, upregulating the voltage-gated sodium channels by promoting their recruitment to the neuronal membrane (149). Furthermore, Albanna et al. showed that moderate UCB levels were able to modify the function of voltage gated Cav2.3 calcium channels, impairing neurotransmission in retinal neurons (150). Moreover, using a mouse model of neonatal hyperbilirubinemia, it was observed that UCB-induced oxidative stress may damage cerebellar DNA (151), which may then contribute to neuronal cell death.
Finally, a link between maternal micronutrients, the nutritional status of the newborn (152) and the deficient enzymatic antioxidant defenses implicated in neuronal damage by UCB (153), may additionally constitute targets for therapeutic interventions in the management of BIND, by potentially exerting protective and regenerative effects.
Gliocentric view of BIND
Besides neurons, it is now recognized that glial cells are relevant players in most neurodegenerative diseases, contributing to the initiation and/or propagation of neuropathological cascades, either by gain or loss of function (154). Actually, glial cells, once considered as the glue between neurons, are presently acknowledged as key players in the brain immune system and in multiple physiological processes linked to synaptic plasticity, energy metabolism, learning and memory formation, among others (155). The intricate balance of homeostatic and inflammatory functions influences the onset and the progression of neurodegenerative diseases (156). Moreover, neurological disorders usually involve feedback loops that disseminate and perpetuate the disease (157), mostly mediated by the cell-secreted soluble factors and release of small (exosomes) and large EVs (158,159), already observed in the cerebrospinal fluid (CSF) of patients with ABE (160). We propose that neuronal selectivity in BIND converges with non-cell autonomous mechanisms involving signaling mechanisms and non-neuronal cell types, thus requiring a better understanding. In this section, we will address data on glial sensitivity to UCB, i.e., the view of a more integrated “gliocentric brain” (161), providing further information on targets to unravel and prevent UCB brain lesions and their sequela, and then assist in the insult recovery.
Myelin damage
The myelinating cells of the CNS, the oligodendrocytes, are generated from bipolar oligodendrocyte progenitor cells (OPCs) that arise between 10 and 18 weeks of gestation in humans (162,163). Maturation of oligodendrocytes start at 28 to 40 weeks of gestation and proceeds during the early postnatal period (141). Oligodendrocytes constitute 5% to 8% of total glial cells (163). The first report on ultrastructural changes in the Gunn rat with bilirubin encephalopathy identified the presence of myelin debris in the cytoplasm of neurons, which also presented mitochondrial alterations and glycogen-filled vacuoles (164). UCB was shown to bind to myelin and was suggested to be associated with its retention in the brain (165,166). However, UCB also caused cerebellar myelin fragmentation in in vitro cultures (167), and myelin loss was observed in biopsy samples from a kernicteric preterm infant (168). Lesions in the myelin sheath of spiral ganglion cells were observed in neonatal guinea pigs exposed to hyperbilirubinemia. Neuroimaging studies in infants at risk for kernicterus identified white matter abnormalities (169). When assessed for in vitro effects, UCB was shown to impair OPCs (Figure 4) (146) and oligodendrocytes (170), as well as to disturb the differentiation of OPCs into myelinating oligodendrocytes (147). Further studies, using rat organotypic cerebellar slices demonstrated that treatment with 20-nM Bf led to a reduction in the number of myelinated fibers, together with the gene expression of the myelin basic protein (171). The data validate that concentrations mimicking neonatal unconjugated hyperbilirubinemia impair myelination. Using a new kernicterus mouse model with Ugt1a1 gene deletion, it was possible to confirm the presence of cerebellum atrophy by the elevated UCB concentrations, together with axonal loss and decreased myelination, which was similarly noticed in the medulla oblongata and pons, but not in the corpus callosum (172). In summary, deficits in myelination should be considered as targets when developing new therapeutic strategies for BIND.
Microglia polarization
Microglia are the resident macrophages of the CNS that are derived from the yolk sac and travel to the brain during early development (173). Microglia represent 5% of total glial cells in the human cortical brain (174) and show phenotypical heterogeneity, regional diversity, and are highly complex and dynamic and with interchangeable phenotypes (175) (Figure 5). Microglia release interleukin (IL)-1β and tumor necrosis factor-alpha (TNF-α), among other cytokines (176), which regulate homeostasis or are involved in neuroinflammation and pathology. Besides phagocytic and pruning functions, microglia regulate myelin uptake, neurogenesis, and cerebral angiogenesis (175). The first data on possible lipid droplet-accumulating microglia were obtained in the Gunn rat cerebellum in 1986 (177), a model of CN1. In conditions leading to HO-1 induction, producing biliverdin from heme (Figure 1), this enzyme was found to be mainly localized in microglia and involved in their activation, but it is unclear whether this might lead to beneficial or harmful effects (178-180). A pioneer study showed that UCB activates microglia leading to the release of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6, as well as glutamate, while also inducing cell death by apoptosis and necrosis (181), suggesting that these cells may have an important role in BIND and, consequently, are promising targets to modulate excessive neuroinflammation. Certain UCB photoproducts also produce neuro-inflammatory effects that may even surpass those of 140-nM Bf (182). Therefore, it is not surprising that microglia are activated after intracerebral hemorrhage and can be associated with bilirubin production in the CNS and its oxidation products (183), while also facilitating early inflammation by neutrophil brain infiltration (184).
Interaction of UCB with microglia first impacts on protective mechanisms associated with the activation of mitogen-activated protein kinases (MAPKs) and nuclear factor kappa B (NF-κB), together with increased phagocytosis, and later release of pro-inflammatory cytokines (185). In early responses to UCB, microglia may then have a protective intervention (185,186). However, in chronic or long-lasting hyperbilirubinemia, such benefits may no longer be supported (187-189). Therefore, the good may turn bad with the release of excessive inflammatory mediators. Actually, microglia are known by their dual neuroprotective and neuroinflammatory roles among the kaleidoscope of polarized phenotypes (190) (Figure 5). In the steady-state, microglia have a ramified morphology with highly motile processes constantly surveying the neighboring environment. Changes in brain homeostasis leads to alterations in microglia shape and process motility. The acquired amoeboid morphology is associated with phagocytic ability and mild inflammation, the rod-shape with activation by mild neurodegeneration, and the hypertrophic with excessive immune reaction. When damaged by chronic insults or senescence, microglia become dystrophic and are ineffective in supporting neural cell homeostasis (191-199). Using transcriptional single-cell sorting, it was possible to identify several immune-related classes and disease-associated microglia (DAM) phenotypes, based on a specific set of genes found in AD models and patients (200-202).
Activation of microglia was observed in the hippocampus and cerebellum of mice with hyperbilirubinemia (172,203,204) and in rat cerebellar slice cultures treated with UCB (171), where the induction of excitotoxic and neurodegenerative processes were identified. However, we still need to better understand microglial population diversity, in which each member may perform unique functions in a disease-context-dependent fashion (205). As already mentioned, senescent microglia associated with aging and AD (192,206,207) show loss of function and chronic release of pro-inflammatory mediators. Caldeira et al. developed an in vitro microglia model able to mimic “young/responsive” and “old/senescent” microglial features (194). These authors using in vitro aging microglia were able to discriminate age-dependent responses by Aβ (195). When such a model was used to assess Bf-induced responses in each of the conditions, increased sickness prevailed in the younger microglia, as compared with the older cells (208), and included enhanced amoeboid morphology, NO release, and elevated high mobility group box protein 1 (HMGB1), TNF-α, and IL-6 gene expression levels. Among the vast number of small non-coding short RNAs (miRNAs) controlling post-transcriptional expression of target genes, some were recognized as inflammatory associated miRNAs (inflamma-miRNAs), and were accepted as key players in microglia function/dysfunction, polarization, and restoration (209). Among those, upregulation of miRNA(miR)-155, miR-125b, miR-21, and miR-146a by Bf was only observed in the “young” microglia, pushing the cell phenotype to an immune-polarized state, and indicating their propensity to be stimulated by Bf. However, Bf seemed to also induce a sort of microglia activation, independent of the age of the cells, based on an induced increased of CD11b staining (associated with a proinflammatory status) and on the elevation of inducible nitric oxide synthase (iNOS) gene expression at a 100-nM concentration. On the contrary, cells behaved differently with early apoptosis exclusively noticed in “younger” microglia, and late apoptosis/necrosis only in “older” cells. Data have shown that microglia reveal age-dependent performance when stimulated by bilirubin, with beneficial and pathological properties that may vary with co-morbidities, CNS region, neurodevelopmental stage, cell maturation, jaundice duration, and hyperbilirubinemia intensity.
Astrocyte aberrancies
Astrocytes comprise nearly 35% of the total CNS population, and like microglia, they may be found in all CNS regions. Astrocytes participate in neuroinflammatory responses and show diverse subtypes that are disorder- and context-specific (210,211). Some of the biomarkers more often used in their characterization are glial fibrillary acidic protein (GFAP), S100B, glutamine synthetase, or the glutamate transporters, GLT1 and GLAST (210,212). One of the first studies using mixed fetal rat glial cells, in which 80% to 95% of cells were astrocytes, identified morphological and cytotoxic alterations, as well as age-in-culture-dependent sensitivity, when working with UCB/HSA ratios of 2, i.e., recapitulating severe hyperbilirubinemia in neonates (213). The study called attention for the higher susceptibility of immature neural cells to UCB harmful effects. The idea was later reinforced by several studies in primary cultures of astrocytes, as well as in neurons and microglia (46,47,140,208,214,215). By using the MTT assay for cell viability and mitochondrial activity assessments, Chuniaud et al. attributed the cytotoxicity of UCB to mitochondria failure (216). Astrocytes have a wide range of relevant functions in the brain that contribute to maintain extracellular homeostasis (217), such as their ability to uptake glutamate, thus preventing its accumulation at synapses and resulting excitotoxicity (218). UCB inhibited glutamate uptake and cell endocytosis, when rat cortical astrocytes were used (219,220). Interestingly, while the inhibition of glutamate was higher in astrocytes than in neurons, cell death and changes in redox stress were prominent in neurons (133,221), suggesting cell pathological susceptibilities. Astrocyte increased resistance may derive from the elevated expression of the multidrug resistance-associated protein 1 (MRP1) that was shown to be promoted by UCB (222).
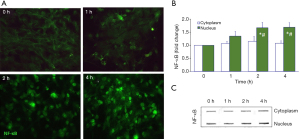
Astrocytes detect infection and injury by neurons, microglia, oligodendrocytes, and endothelial cells, with the secretion of cytokines and growth factors that may act as immune regulators, following the activation of NF-κB. They are accepted as initiators and responders to inflammation, namely to mediators released by the activated microglia, such as IL-1β, TNF-α, and interferon gamma (IFN-γ), and they react to lipopolysaccharide (LPS) (223-226). Interestingly, UCB at 50-µM (UCB/HSA=0.5) caused apoptotic cell death and TNF-α secretion from rat cortical astrocytes, in a similar way to that of 10 ng/mL of LPS. They reacted strongly to UCB, then to LPS, in terms of necrosis, as well as to the secretion of IL-1β and glutamate, and less to the release of IL-6 (227). The cytotoxicity of UCB in astrocytes was also observed with serum from infants with unconjugated hyperbilirubinemia (228). As expected, UCB activated astrocyte signaling pathways associated with MAPKs, i.e., p38, Jun N-terminal kinase (JNK)1⁄2 and extracellular signal-regulated kinase (ERK)1⁄2 pathways, as well as the key player NF-κB (229), which further increased in hypoxia and oxygen-glucose deprivation preconditioning conditions (48). Translocation of NF-κB from the cytoplasm to the nucleus in the cortical astrocytes treated with UCB at 50 µM plus 100-µM HSA was shown to peak at 2 to 4 h of interaction (230) (Figure 6), much later than in microglia, where the peak was observed 30 min after exposure (185), evidencing the early activation of microglia by UCB. We also noticed an induced release of IL-1β and TNF-α from UCB-treated cortical astrocytes with increased expression of the TNF-α receptor (TNFR)1 and IL-1β receptor (IL-1R)1 (231). Moreover, UCB reduced the cytokine pro-forms while activated their converting enzymes, ICE and TACE, respectively (231,232). ICE or caspase-1 activation that leads to pyroptotic cell death (233), pro-inflammatory processes (234), and inflammasome activation, including the regulator of innate immunity NLR family pyrin domain containing 3 (NLRP3) (235), was demonstrated in cultured rat cortical astrocytes after exposure to UCB (236). Astrocytes, acquire phenotypic aberrancies in neurodegenerative diseases and are activated by neuroinflammation and stressful factors, such as UCB, contributing to pathophysiological paracrine signaling events, mainly mediated by EVs containing miRNAs (237-240). In such a way, dysfunctional astrocytes actively contribute to cell homeostatic imbalance in the brain, as will be further explained in the next section.
Dysregulated neuron-glia interplay: the gearbox?
Brain function depends on coordinated interactions and intercellular signals between neurons and glial cells that sustain cell homeostatic balance (241,242). In disease, secretion of pathological signaling molecules and EVs from donor cells determine autocrine and paracrine signaling dysregulation (243,244). Changes in neuro-immune homeostasis and neuroinflammation may start at the neurovascular unit composed by the BBB elements: (I) on one side, the endothelial cells, pericytes, and the astrocyte end foots; and (II) on the other side the glial cells, neurons, and the extracellular matrix. Disruption of BBB by UCB is well documented (120,245-247) and facilitate the entrance of elevated Bf levels into the brain, its interaction with neuronal cells and the emergence of BIND (Figure 1). Disruption of the BBB by bilirubin and hypercarbia/hyperosmolarity (52,248) may also allow the passage of albumin-bound bilirubin (249), though the permeability is higher for the Bf species (250). Within the brain, the binding of Bf to cells is facilitated by acidosis (251), increasing the risk of BIND. Pathological synapse loss and dysfunction depends on the maturation of neuronal circuits, proper function of glial cells, and synaptic refinement (252). By using co-culture systems of dorsal root ganglia neurons from rat embryos with OPCs, it was possible to establish that UCB toxicity on pre-myelinating oligodendrocytes interferes with their maturation and leads to incomplete myelination (147). Considering that oligodendrocytes modulate synaptic transmission through the release of brain-derived neurotrophic factor (BDNF) in the developing brain (253), the delay caused by bilirubin in the maturation of OPCs may then be critical to brain development and BIND disorder.
The concerted activity of neuron-astrocyte communication by neuromodulators, neurotransmitters, glutamate, and calcium (254) is determinant in neurodevelopment and associated diseases (255). It may differ by brain region and with stimulus duration, as observed after injection of bilirubin into the brain (256). Protective astrocyte pathways involve the release of neurotrophic factors, neuropoietic cytokines and a plethora of protective mediators (257), which modulate the propensity of the cells to injury. This was what we observed when the addition of 50-µM UCB plus 100-µM HSA to neuron-astrocyte cocultures did not produce immediate neurotoxic effects (258). Cell recognition triggers pro-survival effects that should have protected cells from UCB injury. Instead, when neuron-astrocyte homeostasis was established for 24 h prior to the addition of the same UCB/HSA molar ratio, then increased neuronal cell death (apoptosis and necrosis), reduced neurite extension and ramification, together with S100B and nitric oxide (NO) release, were then revealed (259).
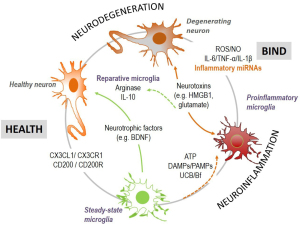
Microglia also modulate CNS homeostasis by: (I) releasing BDNF (that controls neuronal network excitability) (260), secretome functional signaling associated factors and EVs; and (II) bidirectional communication that involves CD200 and fractalkine (CX3CL1) in neurons and the target receptors CD200R and CX3CR1 in microglia (261) (Figure 7). During neuroinflammation by damage-associated molecular patterns (DAMPs), pathogen-associated molecular patterns (PAMPs) and ATP, microglia release pro-inflammatory cytokines (e.g., IL-6, TNF-α, IL-1β), inflamma-miRNAs, reactive oxygen species (ROS) and NO, which may either account to injury repair (262), or to neurodegeneration if excessive (263-265). In such dysregulated homeostasis, increased extracellular neurotoxins, such as HMGB1 and glutamate, further contribute to neuronal dysfunction and microglia overactivation (266). In contrast, reparative/restorative microglia from the integration of pro- and anti-inflammatory mediators, expressing IL-10 and arginase (267,268), intervenes in balancing health and disease. Thus, good or bad cellular environmental conditions may affect the cell response to the UCB stimulus. Indeed, astrocytes may either reduce microglia inflammatory reaction (269), or become more reactive when receiving specific inflammatory mediators from the activated microglia (224), aggravating neuroinflammation, and neurodegeneration. Secretome from UCB-treated astrocytes or neurons, added to UCB-treated microglia modulated IL-1β secretion and enhanced phagocytosis (269), highlighting the benefits of homeostatic cell drivers over the UCB immediate microglia cytotoxicity. Thus, cells establish a very strong communication until attaining homeostasis; but also, their crosstalk after UCB insult may act in a synergistic way to cause a neurotoxic environment. In hippocampal organotypic cultures from Wistar rats at 2 and 8 PN days, harmful effects at PN8 were higher than at PN2 after treatment with 140-nM Bf (144), as well as after 14 days in culture relatively to that observed after only 7 days (270). However, we cannot dismiss the fact that cells may become more susceptible with time in culture. When the effects of microglia in the hippocampus response to UCB was assessed by using microglia-depleted and non-depleted organotypic cultures (PN7-10 plus 72-h slice functional recovery), release of glutamate and NO, as well as cell demise, were higher in the presence of microglia after treatment with 50-µM UCB (at a UCB/HSA =0.5), indicating the joint action of neurons and glial cells in overall nerve cell toxicity surplus (137).
Lately, EVs were shown to be key players in cell-to-cell signaling (209,242,271). These vesicles, besides lipids, proteins, and genetic material, include miRNAs (243) and may release their contents into the extracellular space or into a neighboring cell after fusion or uptake (271), thus sustaining homeostasis or propagating the disease. The small EVs/exosomes produced by neural cells easily cross the BBB and allow reciprocal communication between the CNS and the peripheral circulation, being considered promising biomarkers (272). Profiling of exosomal proteins or miRNAs in serum and CSF showed promise as relevant markers in several brain disorders (273-276). In a recent study, quantitative proteomic characterization of EVs from the CSF of infants with ABE identified the involvement of four proteins associated to immune-inflammation and signaling pathways (160).
In conclusion, neurons and glial cells establish concerted actions that preserve brain function from injury, but also work in a synergistic fashion when the cell homeostatic balance is severely damaged, accounting for the time-dependent aggravating effects of a sustained hyperbilirubinemia. EVs are important players in supporting health or in contributing to disease, and may turn out to be important tools to identify infants at risk of BIND.
Perspectives
Bilirubin encephalopathy and associated KSD have been neglected conditions with limited funding and unsatisfactory health interventions due to insufficient knowledge of the underlying pathological mechanisms. Current available treatment options and potential therapies were recently reviewed (18). Some of the tested interventions include ursodeoxycholic acid (UDCA) or its glycoconjugate (GUDCA) (129,130,139,232,247,277-279), minocycline (280,281), bioactive compounds (282-284), and small-molecule activators (285,286).
Though a few of the strategies were assessed for efficacy in clinical trials (278,279), most were tested in pre-clinical models, from cell cultures to organotypic systems and animal models. Translation of such data to the clinic is a critical challenge that frequently disappoints due to biological discrepancies and different response mechanisms to perturbations among species (287). Reprogramming of dysfunctional neural cells toward pro-regenerative functions, as suggested for microglia (288), may also provide new therapeutic opportunities to prevent excessive UCB-induced neuroinflammation and neurodegeneration. However, strategies able to produce cell revival with modulatory medicines, such as the incorporation of medicines in exosomes, miRNA-based therapies, or cell replacement strategies are innovative approaches that are yet far from being developed or tested in the field of hyperbilirubinemia. The use of fibroblasts from jaundiced infants that can be differentiated into neural cells according to brain regions by reprogramming techniques (induced pluripotent stem cells or iPSCs), or by direct conversion, may also bring new opportunities. These advanced models will be important tools for drug testing, or use in regenerative strategies (e.g., autologous transplantation). Of note, induced hepatocytes from iPSCs transplanted into Gunn rats produced a decline of 30% to 60% of UCB and biliary excretion of bilirubin glucuronides, ameliorating hyperbilirubinemia and showing promise in the treatment of unconjugated inherited liver diseases (289), namely in CN1.
Given the complexity and the multiple factors associated to the risk of BIND or KSD in severe neonatal hyperbilirubinemia, combination of therapeutic strategies might be considered. Circulating exosomes may not only then be used as noninvasive novel biomarkers to help in the clinic, but also for personalized medicine. Engineered exosomes (290), biomimetic exosomes (291), and miRNA-enriched EVs (292), could also contribute to a better cell survival and revival, when used together with the traditional therapeutic interventions, hopefully facilitating neuro-regeneration in infants with bilirubin-associated brain injury.
Acknowledgments
We thank Dr. Adelaide Fernandes Borralho and Dr. Andreia Barateiro for providing images and data included in Figures 4 and 6.
Funding: This work was funded by Fundação para a Ciência e a Tecnologia (FCT) to iMed.ULisboa (UIDB/04138/2020 and UIDP/04138/2020) and to DB (PDTC/MED-NEU/31395/2017 and LISBOA-01-0145-FEDER-031395).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (David K. Stevenson and Ronald J Wong) for the series “Neonatal Jaundice” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review checklist. Available at https://dx.doi.org/10.21037/pm-21-37
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/pm-21-37). The series “Neonatal Jaundice” was commissioned by the editorial office without any funding or sponsorship. DB and RFMS report funding from Fundação para a Ciência e Tecnologia and Fundação para a Ciência e Tecnologia and LISBOA-01-0145-FEDER-031395. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Orth J. Ueber das Vorkommen von Bilirubinkrystallen bei neugeborenen Kindern. Virchows Arch Pathol Anat 1875;63:15. [Crossref]
- Schmorl G. Zur kenntnis des ikterus neonatorum. Verh Dtsch Pathol Ges 1904;6:109-15.
- Qu Y, Huang S, Fu X, et al. Nomogram for acute bilirubin encephalopathy risk in newborns with extreme hyperbilirubinemia. Front Neurol 2020;11:592254. [Crossref] [PubMed]
- Pediatrics AAo. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114:297-316. [Crossref] [PubMed]
- Hameed NN, Hussein MA. BIND score: A system to triage infants readmitted for extreme hyperbilirubinemia. Semin Perinatol 2021;45:151354. [Crossref] [PubMed]
- Capasso L, Palma M, Coppola C, et al. Neonatal hyperbilirubinemia: an updated appraisal of national guidelines. Curr Pediatr Rev 2020;16:298-306. [Crossref] [PubMed]
- Brown SA, Waldrop J, D'Auria J, et al. Improving evaluation and treatment of hyperbilirubinemia in late preterm infants. J Perinat Neonatal Nurs 2020;34:346-51. [Crossref] [PubMed]
- Zhang M, Tang J, He Y, et al. Systematic review of global clinical practice guidelines for neonatal hyperbilirubinemia. BMJ Open 2021;11:e040182. [Crossref] [PubMed]
- Iskander I, Gamaleldin R. Acute bilirubin encephalopathy: some lessons learned. Semin Perinatol 2021;45:151353. [Crossref] [PubMed]
- Ahmad M, Rehman A, Adnan M, et al. Acute bilirubin encephalopathy and its associated risk factors in a tertiary care hospital, Pakistan. Pak J Med Sci 2020;36:1189-92. [Crossref] [PubMed]
- Farouk ZL, Slusher TM, Danzomo AA, et al. Knowledge, Observation and practices related to neonatal jaundice in a rural community in Kano, Nigeria. J Trop Pediatr 2021;67:fmaa134.
- Erdeve O. Management of neonatal jaundice in low-income and middle-income countries. BMJ Paediatr Open 2020;4:e000845. [Crossref] [PubMed]
- Mir SE, van der Geest BAM, Been JV. Management of neonatal jaundice in low- and lower-middle-income countries. BMJ Paediatr Open 2019;3:e000408. [Crossref] [PubMed]
- Slusher TM, Vaucher YE. Management of neonatal jaundice in low- and middle-income countries. Paediatr Int Child Health 2020;40:7-10. [Crossref] [PubMed]
- Ahlfors CE, Wennberg RP, Ostrow JD, et al. Unbound (free) bilirubin: improving the paradigm for evaluating neonatal jaundice. Clin Chem 2009;55:1288-99. [Crossref] [PubMed]
- Riordan SM, Shapiro SM. Review of bilirubin neurotoxicity I: molecular biology and neuropathology of disease. Pediatr Res 2020;87:327-31. [Crossref] [PubMed]
- Hansen TWR, Wong RJ, Stevenson DK. Molecular physiology and pathophysiology of bilirubin handling by the blood, liver, intestine, and brain in the newborn. Physiol Rev 2020;100:1291-346. [Crossref] [PubMed]
- Shapiro SM, Riordan SM. Review of bilirubin neurotoxicity II: preventing and treating acute bilirubin encephalopathy and kernicterus spectrum disorders. Pediatr Res 2020;87:332-7. [Crossref] [PubMed]
- Arain Y, Banda JM, Faulkenberry J, et al. Clinical decision support tool for phototherapy initiation in preterm infants. J Perinatol 2020;40:1518-23. [Crossref] [PubMed]
- Anderson NB, Calkins KL. Neonatal indirect hyperbilirubinemia. Neoreviews 2020;21:e749-60. [Crossref] [PubMed]
- Singh A, Jialal I. Unconjugated hyperbilirubinemia. StatPearls. Treasure Island (FL) 2020.
- Porter ML, Dennis BL. Hyperbilirubinemia in the term newborn. Am Fam Physician 2002;65:599-606. [PubMed]
- Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008;371:64-74. [Crossref] [PubMed]
- Kaplan M. Genetic interactions in the pathogenesis of neonatal hyperbilirubinemia: Gilbert's Syndrome and glucose-6-phosphate dehydrogenase deficiency. J Perinatol 2001;21:S30-4; discussion S35-9. [Crossref] [PubMed]
- Luzzatto L, Ally M, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Blood 2020;136:1225-40. [Crossref] [PubMed]
- Kaplan M, Wong RJ, Stevenson DK. Hemolysis and glucose-6-phosphate dehydrogenase deficiency-related neonatal hyperbilirubinemia. Neonatology 2018;114:223-5. [Crossref] [PubMed]
- Kaplan M, Hammerman C, Bhutani VK. The Preterm infant: a high-risk situation for neonatal hyperbilirubinemia due to glucose-6-phosphate dehydrogenase deficiency. Clin Perinatol 2016;43:325-40. [Crossref] [PubMed]
- Tayyab S, Ali MK. Binding of bilirubin to mammalian erythrocytes. Comp Biochem Physiol B Biochem Mol Biol 1997;118:97-103. [Crossref] [PubMed]
- Bratlid D. Bilirubin binding by human erythrocytes. Scand J Clin Lab Invest 1972;29:91-7. [Crossref] [PubMed]
- Brites D, Silva R, Brito A. Effect of bilirubin on erythrocyte shape and haemolysis, under hypotonic, aggregating or non-aggregating conditions, and correlation with cell age. Scand J Clin Lab Invest 1997;57:337-49. [Crossref] [PubMed]
- Brito MA, Brites D. Effect of acidosis on bilirubin-induced toxicity to human erythrocytes. Mol Cell Biochem 2003;247:155-62. [Crossref] [PubMed]
- Brito MA, Silva RF, Brites D. Bilirubin induces loss of membrane lipids and exposure of phosphatidylserine in human erythrocytes. Cell Biol Toxicol 2002;18:181-92. [Crossref] [PubMed]
- Brito MA, Silva R, Tiribelli C, et al. Assessment of bilirubin toxicity to erythrocytes. Implication in neonatal jaundice management. Eur J Clin Invest 2000;30:239-47. [Crossref] [PubMed]
- Memon N, Weinberger BI, Hegyi T, et al. Inherited disorders of bilirubin clearance. Pediatr Res 2016;79:378-86. [Crossref] [PubMed]
- Li Z, Song L, Hao L. The role of UGT1A1 (c.-3279 T > G) gene polymorphisms in neonatal hyperbilirubinemia susceptibility. BMC Med Genet 2020;21:218. [Crossref] [PubMed]
- Bhandari J, Thada PK, Yadav D. Crigler Najjar Syndrome. StatPearls. Treasure Island (FL) 2020.
- Strauss KA, Ahlfors CE, Soltys K, et al. Crigler-Najjar Syndrome Type 1: pathophysiology, natural history, and therapeutic frontier. Hepatology 2020;71:1923-39. [Crossref] [PubMed]
- Bartlett MG, Gourley GR. Assessment of UGT polymorphisms and neonatal jaundice. Semin Perinatol 2011;35:127-33. [Crossref] [PubMed]
- van der Veere CN, Sinaasappel M, McDonagh AF, et al. Current therapy for Crigler-Najjar syndrome type 1: report of a world registry. Hepatology 1996;24:311-5. [Crossref] [PubMed]
- Čvorović J, Passamonti S. Membrane transporters for bilirubin and its conjugates: a systematic review. Front Pharmacol 2017;8:887. [Crossref] [PubMed]
- De Carvalho M, Robertson S, Klaus M. Fecal bilirubin excretion and serum bilirubin concentrations in breast-fed and bottle-fed infants. J Pediatr 1985;107:786-90. [Crossref] [PubMed]
- Venigalla S, Gourley GR. Neonatal cholestasis. Semin Perinatol 2004;28:348-55. [Crossref] [PubMed]
- Watchko JF. Bilirubin-induced neurotoxicity in the preterm neonate. Clin Perinatol 2016;43:297-311. [Crossref] [PubMed]
- Stevenson DK, Bhutani VK. Preterm neonates: beyond the guidelines for neonatal hyperbilirubinemia. Clin Perinatol 2016;43:xvii-xviii. [Crossref] [PubMed]
- Bhutani VK, Wong RJ, Stevenson DK. Hyperbilirubinemia in preterm neonates. Clin Perinatol 2016;43:215-32. [Crossref] [PubMed]
- Falcão AS, Fernandes A, Brito MA, et al. Bilirubin-induced inflammatory response, glutamate release, and cell death in rat cortical astrocytes are enhanced in younger cells. Neurobiol Dis 2005;20:199-206. [Crossref] [PubMed]
- Falcão AS, Fernandes A, Brito MA, et al. Bilirubin-induced immunostimulant effects and toxicity vary with neural cell type and maturation state. Acta Neuropathol 2006;112:95-105. [Crossref] [PubMed]
- Falcão AS, Silva RF, Fernandes A, et al. Influence of hypoxia and ischemia preconditioning on bilirubin damage to astrocytes. Brain Res 2007;1149:191-9. [Crossref] [PubMed]
- Odutolu Y, Emmerson AJ. Low bilirubin kernicterus with sepsis and hypoalbuminaemia. BMJ Case Rep 2013;2013:bcr2012008042. [Crossref] [PubMed]
- Watchko JF, Spitzer AR, Clark RH. Prevalence of hypoalbuminemia and elevated bilirubin/albumin ratios in a large cohort of infants in the neonatal intensive care unit. J Pediatr 2017;188:280-6.e4. [Crossref] [PubMed]
- Perlman M, Kapitulnik J, Blondheim SH, et al. Bilirubin binding and neonatal acidosis. Clin Chem 1981;27:1872-4. [Crossref] [PubMed]
- Bratlid D, Cashore WJ, Oh W. Effect of acidosis on bilirubin deposition in rat brain. Pediatrics 1984;73:431-4. [PubMed]
- Maisels JMW, Jaundice JF. In: MacDonald MGS, Mary MK. editors. Avery's Neonatology: Pathophysiology & Management of the Newborn, 7th ed. Lippincott Williams & Wilkins, 2015:1216.
- Bratton S, Cantu RM, Stern M. Breast milk jaundice. StatPearls. Treasure Island (FL) 2020.
- Li Y, Shen N, Li J, et al. Changes in intestinal flora and metabolites in neonates with breast milk jaundice. Front Pediatr 2020;8:177. [Crossref] [PubMed]
- Duan M, Han ZH, Huang T, et al. Characterization of gut microbiota and short-chain fatty acid in breastfed infants with or without breast milk jaundice. Lett Appl Microbiol 2021;72:60-7. [Crossref] [PubMed]
- Chen K, Yuan T. The role of microbiota in neonatal hyperbilirubinemia. Am J Transl Res 2020;12:7459-74. [PubMed]
- Farouk ZL, Usman F, Musa BM, et al. Societal awareness on neonatal hyperbilirubinemia: a systematic review and meta-analysis. Semin Perinatol 2021;45:151361. [Crossref] [PubMed]
- Watchko JF. TcB, FFR, phototherapy and the persistent occurrence of kernicterus spectrum disorder. J Perinatol 2020;40:177-9. [Crossref] [PubMed]
- Stocker R, Yamamoto Y, Mcdonagh AF, et al. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043-6. [Crossref] [PubMed]
- Ostrow JD, Tiribelli C. Bilirubin, a curse and a boon. Gut 2003;52:1668-70. [Crossref] [PubMed]
- Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci 2009;30:129-37. [Crossref] [PubMed]
- Maghzal GJ, Leck MC, Collinson E, et al. Limited role for the bilirubin-biliverdin redox amplification cycle in the cellular antioxidant protection by biliverdin reductase. J Biol Chem 2009;284:29251-9. [Crossref] [PubMed]
- Barone E, Di Domenico F, Mancuso C, et al. The Janus face of the heme oxygenase/biliverdin reductase system in Alzheimer disease: it's time for reconciliation. Neurobiol Dis 2014;62:144-59. [Crossref] [PubMed]
- Jangi S, Otterbein L, Robson S. The molecular basis for the immunomodulatory activities of unconjugated bilirubin. Int J Biochem Cell Biol 2013;45:2843-51. [Crossref] [PubMed]
- Kim TW, Kim Y, Jung W, et al. Bilirubin nanomedicine ameliorates the progression of experimental autoimmune encephalomyelitis by modulating dendritic cells. J Control Release 2021;331:74-84. [Crossref] [PubMed]
- Li Y, Huang B, Ye T, et al. Physiological concentrations of bilirubin control inflammatory response by inhibiting NF-kappaB and inflammasome activation. Int Immunopharmacol 2020;84:106520. [Crossref] [PubMed]
- Bianco A, Dvorak A, Capkova N, et al. The extent of intracellular accumulation of bilirubin determines its anti- or pro-oxidant effect. Int J Mol Sci 2020;21:8101. [Crossref] [PubMed]
- Jayanti S, Vitek L, Tiribelli C, et al. The role of bilirubin and the other "yellow players" in neurodegenerative diseases. Antioxidants (Basel) 2020;9:900. [Crossref] [PubMed]
- Jayanti S, Moretti R, Tiribelli C, et al. Bilirubin and inflammation in neurodegenerative and other neurological diseases. Neuroimmunol Neuroinflamm 2020;7:92-108. [Crossref]
- Zahir F, Rabbani G, Khan RH, et al. The pharmacological features of bilirubin: the question of the century. Cell Mol Biol Lett 2015;20:418-47. [Crossref] [PubMed]
- Wagner KH, Wallner M, Molzer C, et al. Looking to the horizon: the role of bilirubin in the development and prevention of age-related chronic diseases. Clin Sci (Lond) 2015;129:1-25. [Crossref] [PubMed]
- Vitek L, Bellarosa C, Tiribelli C. Induction of mild hyperbilirubinemia: hype or real therapeutic opportunity? Clin Pharmacol Ther 2019;106:568-75. [Crossref] [PubMed]
- Chen Z, Vong CT, Gao C, et al. Bilirubin nanomedicines for the treatment of reactive oxygen species (ros)-mediated diseases. Mol Pharm 2020;17:2260-74. [Crossref] [PubMed]
- Alkén J, Hakansson S, Ekeus C, et al. Rates of extreme neonatal hyperbilirubinemia and kernicterus in children and adherence to national guidelines for screening, diagnosis, and treatment in Sweden. JAMA Netw Open 2019;2:e190858. [Crossref] [PubMed]
- Koziol LF, Budding DE, Chidekel D. Hyperbilirubinemia: subcortical mechanisms of cognitive and behavioral dysfunction. Pediatr Neurol 2013;48:3-13. [Crossref] [PubMed]
- Johnson L, Bhutani VK. The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Perinatol 2011;35:101-13. [Crossref] [PubMed]
- Jenabi E, Bashirian S, Khazaei S. Association between neonatal jaundice and autism spectrum disorders among children: a meta-analysis. Clin Exp Pediatr 2020;63:8-13. [Crossref] [PubMed]
- Amin SB, Smith T, Wang H. Is neonatal jaundice associated with Autism Spectrum Disorders: a systematic review. J Autism Dev Disord 2011;41:1455-63. [Crossref] [PubMed]
- Jiang ZD, Wilkinson AR. Impaired function of the auditory brainstem in term neonates with hyperbilirubinemia. Brain Dev 2014;36:212-8. [Crossref] [PubMed]
- Ezzeldin ZM, Sharaf E, Hamdy HS, et al. Hearing screening in neonates with hyperbilirubinemia. Int J Pediatr Otorhinolaryngol 2021;142:110591. [Crossref] [PubMed]
- Johnson L, Brown A, Bhutani V. BIND-a clinical score for bilirubin induced neurologic dysfunction in newborns. Pediatrics 1999;104:746-7.
- Shapiro SM. Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (BIND). J Perinatol 2005;25:54-9. [Crossref] [PubMed]
- Olds C, Oghalai JS. Bilirubin-induced audiologic injury in preterm infants. Clin Perinatol 2016;43:313-23. [Crossref] [PubMed]
- Lunsing RJ. Subtle bilirubin-induced neurodevelopmental dysfunction (BIND) in the term and late preterm infant: does it exist? Semin Perinatol 2014;38:465-71. [Crossref] [PubMed]
- Good WV, Hou C. Visuocortical bilirubin-induced neurological dysfunction. Semin Fetal Neonatal Med 2015;20:37-41. [Crossref] [PubMed]
- Agrawal A, Pandya S, Shrivastava J. Neurodevelopmental outcome at 6 months of age in full-term healthy newborns with neonatal hyperbilirubinemia. J Clin Neonatol 2020;9:138. [Crossref]
- Dubey P, Shrivastava J, Choubey BP, et al. Neurodevelopmental outcome of healthy term newborn with serum bilirubin >15 mg/dl at one year. J Neonatal Perinatal Med 2021;14:339-44. [Crossref] [PubMed]
- Scheidt PC, Graubard BI, Nelson KB, et al. Intelligence at six years in relation to neonatal bilirubin levels: follow-up of the National Institute of Child Health and Human Development Clinical Trial of Phototherapy. Pediatrics 1991;87:797-805. [PubMed]
- Newman TB, Klebanoff MA. Neonatal hyperbilirubinemia and long-term outcome: another look at the Collaborative Perinatal Project. Pediatrics 1993;92:651-7. [PubMed]
- Ebbesen F, Ehrenstein V, Traeger M, et al. Neonatal non-hemolytic hyperbilirubinemia: a prevalence study of adult neuropsychiatric disability and cognitive function in 463 male Danish conscripts. Arch Dis Child 2010;95:583-7. [Crossref] [PubMed]
- Newman TB, Liljestrand P, Jeremy RJ, et al. Outcomes among newborns with total serum bilirubin levels of 25 mg per deciliter or more. N Engl J Med 2006;354:1889-900. [Crossref] [PubMed]
- Hansen RL, Hughes GG, Ahlfors CE. Neonatal bilirubin exposure and psychoeducational outcome. J Dev Behav Pediatr 1991;12:287-93. [Crossref] [PubMed]
- Hokkanen L, Launes J, Michelsson K. Adult neurobehavioral outcome of hyperbilirubinemia in full term neonates-a 30 year prospective follow-up study. PeerJ 2014;2:e294. [Crossref] [PubMed]
- Chen H, Liang L, Xu H, et al. Short term exposure to bilirubin induces encephalopathy similar to Alzheimer's disease in late life. J Alzheimers Dis 2020;73:277-95. [Crossref] [PubMed]
- Zhong X, Liao Y, Chen X, et al. Abnormal serum bilirubin/albumin concentrations in dementia patients with abeta deposition and the benefit of intravenous albumin infusion for Alzheimer's disease treatment. Front Neurosci 2020;14:859. [Crossref] [PubMed]
- Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med 2010;15:157-63. [Crossref] [PubMed]
- Wusthoff CJ, Loe IM. Impact of bilirubin-induced neurologic dysfunction on neurodevelopmental outcomes. Semin Fetal Neonatal Med 2015;20:52-7. [Crossref] [PubMed]
- Lunsing RJ, Pardoen WF, Hadders-Algra M. Neurodevelopment after moderate hyperbilirubinemia at term. Pediatr Res 2013;73:655-60. [Crossref] [PubMed]
- Pieragnoli E. New concepts on the physiopathology of the bile pigments; pathogenesis of anhemolytic jaundice due to free bilirubin. G Clin Med 1958;39:1944-50. [PubMed]
- Fauvert R, Benhamou JP. Types of jaundice with free bilirubin. Rev Prat 1960;10:831-50. [PubMed]
- Jacobsen J. Binding of bilirubin to human serum albumin - determination of the dissociation constants. FEBS Lett 1969;5:112-4. [Crossref] [PubMed]
- Brodersen R, Funding L, Pedersen AO, et al. Binding of bilirubin to low-affinity sites of human serum albumin in vitro followed by co-crystallization. Scand J Clin Lab Invest 1972;29:433-46. [Crossref] [PubMed]
- Schiff D, Chan G, Stern L. Sephadex G-25 quantitative estimation of free bilirubin potential in jaundiced newborn infants sera: a guide to the prevention of kernicterus. J Lab Clin Med 1972;80:455-62. [PubMed]
- Kapitulnik J, Blondheim SH, Kaufmann NA. Sephadex absorption of bilirubin from neonatal and adult serum. Clin Chem 1972;18:43-7. [Crossref] [PubMed]
- Brodersen R. Competitive binding of bilirubin and drugs to human serum albumin studied by enzymatic oxidation. J Clin Invest 1974;54:1353-64. [Crossref] [PubMed]
- Kapitulnik J, Valaes T, Kaufmann NA, et al. Clinical evaluation of Sephadex gel filtration in estimation of bilirubin binding in serum in neonatal jaundice. Arch Dis Child 1974;49:886-94. [Crossref] [PubMed]
- Knight CG. Chromogenic substrates for proteinases. Purification by chromatography on lipophilic sephadex. J Chromatogr 1976;123:429-33. [Crossref] [PubMed]
- Cashore WJ, Monin PJ, Oh W. Serum bilirubin binding capacity and free bilirubin concentration: a comparison between sephadex G-25 filtration and peroxidase oxidation techniques. Pediatr Res 1978;12:195-8. [Crossref] [PubMed]
- Wennberg RP, Ahlfors CE, Rasmussen LF. The pathochemistry of kernicterus. Early Hum Dev 1979;3:353-72. [Crossref] [PubMed]
- Ostrow JD, Pascolo L, Shapiro SM, et al. New concepts in bilirubin encephalopathy. Eur J Clin Invest 2003;33:988-97. [Crossref] [PubMed]
- Moreau-Clevede J, Pays M. Erythrocyte bilirubin determination (author's transl). Ann Biol Clin (Paris) 1979;37:95-101. [PubMed]
- Krakaur RB, Scanlon JW. Detection of free bilirubin and estimation of reserve albumin binding capacity. Clin Lab Med 1981;1:329-43. [Crossref] [PubMed]
- Brito MA, Silva RM, Matos DC, et al. Alterations of erythrocyte morphology and lipid composition by hyperbilirubinemia. Clin Chim Acta 1996;249:149-65. [Crossref] [PubMed]
- Odell GB, Cukier JO, Ostrea EM Jr, et al. The influence of fatty acids on the binding of bilirubin to albumin. J Lab Clin Med 1977;89:295-307. [PubMed]
- Ballowitz L. Displacement of bilirubin from albumin by drugs. J Pediatr 1978;92:166-7. [Crossref] [PubMed]
- Rasmussen LF, Ahlfors CE, Wennberg RP. Displacement of bilirubin from albumin by indomethacin. J Clin Pharmacol 1978;18:477-81. [Crossref] [PubMed]
- Ivarsen R, Brodersen R. Displacement of bilirubin from adult and newborn serum albumin by a drug and fatty acid. Dev Pharmacol Ther 1989;12:19-29. [Crossref] [PubMed]
- Diamond I, Schmid R. Experimental bilirubin encephalopathy. The mode of entry of bilirubin-14C into the central nervous system. J Clin Invest 1966;45:678-89. [Crossref] [PubMed]
- Cardoso FL, Kittel A, Veszelka S, et al. Exposure to lipopolysaccharide and/or unconjugated bilirubin impair the integrity and function of brain microvascular endothelial cells. PLoS One 2012;7:e35919. [Crossref] [PubMed]
- Palmela I, Sasaki H, Cardoso FL, et al. Time-dependent dual effects of high levels of unconjugated bilirubin on the human blood-brain barrier lining. Front Cell Neurosci 2012;6:22. [Crossref] [PubMed]
- Brito MA, Palmela I, Cardoso FL, et al. Blood-brain barrier and bilirubin: clinical aspects and experimental data. Arch Med Res 2014;45:660-76. [Crossref] [PubMed]
- Chen HC, Wang CH, Tsan KW, et al. An electron microscopic and radioautographic study on experimental kernicterus. II. Bilirubin movement within neurons and release of waste products via astroglia Am J Pathol 1971;64:45-66. [PubMed]
- Mustafa MG, Cowger ML, King TE. Effects of bilirubin on mitochondrial reactions. J Biol Chem 1969;244:6403-14. [Crossref] [PubMed]
- Ernster L, Zetterstrom R. Bilirubin, an uncoupler of oxidative phosphorylation in isolated mitochondria. Nature 1956;178:1335-7. [Crossref] [PubMed]
- Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 2010;11:872-84. [Crossref] [PubMed]
- Liu YJ, McIntyre RL, Janssens GE, et al. Mitochondrial fission and fusion: a dynamic role in aging and potential target for age-related disease. Mech Ageing Dev 2020;186:111212. [Crossref] [PubMed]
- Tilokani L, Nagashima S, Paupe V, et al. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem 2018;62:341-60. [Crossref] [PubMed]
- Rodrigues CM, Solá S, Brites D. Bilirubin induces apoptosis via the mitochondrial pathway in developing rat brain neurons. Hepatology 2002;35:1186-95. [Crossref] [PubMed]
- Silva RF, Rodrigues CM, Brites D. Bilirubin-induced apoptosis in cultured rat neural cells is aggravated by chenodeoxycholic acid but prevented by ursodeoxycholic acid. J Hepatol 2001;34:402-8. [Crossref] [PubMed]
- Ostrow JD, Pascolo L, Brites D, et al. Molecular basis of bilirubin-induced neurotoxicity. Trends Mol Med 2004;10:65-70. [Crossref] [PubMed]
- Hankø E, Hansen TW, Almaas R, et al. Bilirubin induces apoptosis and necrosis in human NT2-N neurons. Pediatr Res 2005;57:179-84. [Crossref] [PubMed]
- Silva RF, Rodrigues CM, Brites D. Rat cultured neuronal and glial cells respond differently to toxicity of unconjugated bilirubin. Pediatr Res 2002;51:535-41. [Crossref] [PubMed]
- Grojean S, Koziel V, Vert P, et al. Bilirubin induces apoptosis via activation of NMDA receptors in developing rat brain neurons. Exp Neurol 2000;166:334-41. [Crossref] [PubMed]
- Brites D. Bilirubin injury to neurons and glial cells: new players, novel targets, and newer insights. Semin Perinatol 2011;35:114-20. [Crossref] [PubMed]
- Brites D. The evolving landscape of neurotoxicity by unconjugated bilirubin: role of glial cells and inflammation. Front Pharmacol 2012;3:88. [Crossref] [PubMed]
- Silva SL, Vaz AR, Diogenes MJ, et al. Neuritic growth impairment and cell death by unconjugated bilirubin is mediated by NO and glutamate, modulated by microglia, and prevented by glycoursodeoxycholic acid and interleukin-10. Neuropharmacology 2012;62:2398-408. [Crossref] [PubMed]
- Mancuso C. Bilirubin and brain: a pharmacological approach. Neuropharmacology 2017;118:113-23. [Crossref] [PubMed]
- Vaz AR, Delgado-Esteban M, Brito MA, et al. Bilirubin selectively inhibits cytochrome c oxidase activity and induces apoptosis in immature cortical neurons: assessment of the protective effects of glycoursodeoxycholic acid. J Neurochem 2010;112:56-65. [Crossref] [PubMed]
- Rodrigues CM, Solá S, Silva RF, et al. Aging confers different sensitivity to the neurotoxic properties of unconjugated bilirubin. Pediatr Res 2002;51:112-8. [Crossref] [PubMed]
- Brites D, Fernandes A. Bilirubin-induced neural impairment: a special focus on myelination, age-related windows of susceptibility and associated co-morbidities. Semin Fetal Neonatal Med 2015;20:14-9. [Crossref] [PubMed]
- Ostrow JD, Pascolo L, Tiribelli C. Reassessment of the unbound concentrations of unconjugated bilirubin in relation to neurotoxicity in vitro. Pediatr Res 2003;54:926. [Crossref] [PubMed]
- Vaz AR, Silva SL, Barateiro A, et al. Selective vulnerability of rat brain regions to unconjugated bilirubin. Mol Cell Neurosci 2011;48:82-93. [Crossref] [PubMed]
- Dal Ben M, Bottin C, Zanconati F, et al. Evaluation of region selective bilirubin-induced brain damage as a basis for a pharmacological treatment. Sci Rep 2017;7:41032. [Crossref] [PubMed]
- Qaisiya M, Brischetto C, Jasprova J, et al. Bilirubin-induced ER stress contributes to the inflammatory response and apoptosis in neuronal cells. Arch Toxicol 2017;91:1847-58. [Crossref] [PubMed]
- Barateiro A, Vaz AR, Silva SL, et al. ER stress, mitochondrial dysfunction and calpain/JNK activation are involved in oligodendrocyte precursor cell death by unconjugated bilirubin. Neuromolecular Med 2012;14:285-302. [Crossref] [PubMed]
- Barateiro A, Miron VE, Santos SD, et al. Unconjugated bilirubin restricts oligodendrocyte differentiation and axonal myelination. Mol Neurobiol 2013;47:632-44. [Crossref] [PubMed]
- Kitchen ST, Tang N, He M, et al. Bilirubin inhibits lipid raft dependent functions of L1 cell adhesion molecule in rat pup cerebellar granule neurons. Pediatr Res 2021;89:1389-95. [Crossref] [PubMed]
- Shi HS, Lai K, Yin XL, et al. Ca(2+)-dependent recruitment of voltage-gated sodium channels underlies bilirubin-induced overexcitation and neurotoxicity. Cell Death Dis 2019;10:774. [Crossref] [PubMed]
- Albanna W, Luke JN, Schubert GA, et al. Modulation of Cav2.3 channels by unconjugated bilirubin (UCB) - Candidate mechanism for UCB-induced neuromodulation and neurotoxicity. Mol Cell Neurosci 2019;96:35-46. [Crossref] [PubMed]
- Rawat V, Bortolussi G, Gazzin S, et al. Bilirubin-induced oxidative stress leads to dna damage in the cerebellum of hyperbilirubinemic neonatal mice and activates DNA double-strand break repair pathways in human cells. Oxid Med Cell Longev 2018;2018:1801243. [Crossref] [PubMed]
- Roy S, Sable P, Khaire A, et al. Effect of maternal micronutrients (folic acid and vitamin B12) and omega 3 fatty acids on indices of brain oxidative stress in the offspring. Brain Dev 2014;36:219-27. [Crossref] [PubMed]
- Bortolussi G, Codarin E, Antoniali G, et al. Impairment of enzymatic antioxidant defenses is associated with bilirubin-induced neuronal cell death in the cerebellum of Ugt1 KO mice. Cell Death Dis 2015;6:e1739. [Crossref] [PubMed]
- Verkhratsky A, Sofroniew MV, Messing A, et al. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro 2012;4:e00082. [Crossref] [PubMed]
- Stevenson R, Samokhina E, Rossetti I, et al. Neuromodulation of glial function during neurodegeneration. Front Cell Neurosci 2020;14:278. [Crossref] [PubMed]
- Sheeler C, Rosa JG, Ferro A, et al. Glia in neurodegeneration: the housekeeper, the defender and the perpetrator. Int J Mol Sci 2020;21:9188. [Crossref] [PubMed]
- Gleichman AJ, Carmichael ST. Glia in neurodegeneration: drivers of disease or along for the ride? Neurobiol Dis 2020;142:104957. [Crossref] [PubMed]
- Hill AF. Extracellular vesicles and neurodegenerative diseases. J Neurosci 2019;39:9269-73. [Crossref] [PubMed]
- Hwang I. Cell-cell communication via extracellular membrane vesicles and its role in the immune response. Mol Cells 2013;36:105-11. [Crossref] [PubMed]
- Tan N, Hu S, Hu Z, et al. Quantitative proteomic characterization of microvesicles/exosomes from the cerebrospinal fluid of patients with acute bilirubin encephalopathy. Mol Med Rep 2020;22:1257-68. [Crossref] [PubMed]
- Robertson JM. The gliocentric brain. Int J Mol Sci 2018;19:3033. [Crossref] [PubMed]
- Barateiro A, Brites D, Fernandes A. Oligodendrocyte development and myelination in neurodevelopment: molecular mechanisms in health and disease. Curr Pharm Des 2016;22:656-79. [Crossref] [PubMed]
- Kuhn S, Gritti L, Crooks D, et al. Oligodendrocytes in development, myelin generation and beyond. Cells 2019;8:1424. [Crossref] [PubMed]
- Jew JY, Williams TH. Ultrastructural aspects of bilirubin encephalopathy in cochlear nuclei of the Gunn rat. J Anat 1977;124:599-614. [PubMed]
- Gurba PE, Zand R. Bilirubin binding to myelin basic protein, histones and its inhibition in vitro of cerebellar protein synthesis. Biochem Biophys Res Commun 1974;58:1142-7. [Crossref] [PubMed]
- Hansen T, Tommarello S, Allen J. Subcellular localization of bilirubin in rat brain after in vivo i.v. administration of [3H]bilirubin. Pediatr Res 2001;49:203-7. [Crossref] [PubMed]
- Silberberg DH, Schutta HS. The effects of unconjugated bilirubin and related pigments on cultures of rat cerebellum. J Neuropathol Exp Neurol 1967;26:572-83. [Crossref] [PubMed]
- Brito MA, Zurolo E, Pereira P, et al. Cerebellar axon/myelin loss, angiogenic sprouting, and neuronal increase of vascular endothelial growth factor in a preterm infant with kernicterus. J Child Neurol 2012;27:615-24. [Crossref] [PubMed]
- Gkoltsiou K, Tzoufi M, Counsell S, et al. Serial brain MRI and ultrasound findings: relation to gestational age, bilirubin level, neonatal neurologic status and neurodevelopmental outcome in infants at risk of kernicterus. Early Hum Dev 2008;84:829-38. [Crossref] [PubMed]
- Genc S, Genc K, Kumral A, et al. Bilirubin is cytotoxic to rat oligodendrocytes in vitro. Brain Res 2003;985:135-41. [Crossref] [PubMed]
- Barateiro A, Domingues HS, Fernandes A, et al. Rat cerebellar slice cultures exposed to bilirubin evidence reactive gliosis, excitotoxicity and impaired myelinogenesis that is prevented by AMPA and TNF-alpha inhibitors. Mol Neurobiol 2014;49:424-39. [Crossref] [PubMed]
- Barateiro A, Chen S, Yueh MF, et al. Reduced myelination and increased glia reactivity resulting from severe neonatal hyperbilirubinemia. Mol Pharmacol 2016;89:84-93. [Crossref] [PubMed]
- Brites D, Bhutani VK. Pathways involving bilirubin and other brain-injuring agents In: Bernard D, Mayston M, Paneth N et al., editors. Cerebral Palsy; Science and Clinical Practice. London: Mac Keith Press; 2014. p. 131-50.
- Pelvig DP, Pakkenberg H, Stark AK, et al. Neocortical glial cell numbers in human brains. Neurobiol Aging 2008;29:1754-62. [Crossref] [PubMed]
- Tan YL, Yuan Y, Tian L. Microglial regional heterogeneity and its role in the brain. Mol Psychiatry 2020;25:351-67. [Crossref] [PubMed]
- Kawasaki Y, Zhang L, Cheng JK, et al. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 2008;28:5189-94. [Crossref] [PubMed]
- Keino H, Aoki E, Kashiwamata S. Postnatal changes of the number and lobular distribution of acid phosphatase positive and lipid granule-containing cells in the cerebellum of hyperbilirubinemic Gunn rats. Neurosci Res 1986;3:183-95. [Crossref] [PubMed]
- Matz P, Turner C, Weinstein PR, et al. Heme-oxygenase-1 induction in glia throughout rat brain following experimental subarachnoid hemorrhage. Brain Res 1996;713:211-22. [Crossref] [PubMed]
- Matsuoka Y, Kitamura Y, Okazaki M, et al. Kainic acid induction of heme oxygenase in vivo and in vitro. Neuroscience 1998;85:1223-33. [Crossref] [PubMed]
- Matsuoka Y, Okazaki M, Kitamura Y. Induction of inducible heme oxygenase (HO-1) in the central nervous system: is HO-1 helpful or harmful? Neurotox Res 1999;1:113-7. [Crossref] [PubMed]
- Gordo AC, Falcão AS, Fernandes A, et al. Unconjugated bilirubin activates and damages microglia. J Neurosci Res 2006;84:194-201. [Crossref] [PubMed]
- Jašprová J, Dal Ben M, Hurny D, et al. Neuro-inflammatory effects of photodegradative products of bilirubin. Sci Rep 2018;8:7444. [Crossref] [PubMed]
- Loftspring MC, Hansen C, Clark JF. A novel brain injury mechanism after intracerebral hemorrhage: the interaction between heme products and the immune system. Med Hypotheses 2010;74:63-6. [Crossref] [PubMed]
- Loftspring MC, Johnson HL, Feng R, et al. Unconjugated bilirubin contributes to early inflammation and edema after intracerebral hemorrhage. J Cereb Blood Flow Metab 2011;31:1133-42. [Crossref] [PubMed]
- Silva SL, Vaz AR, Barateiro A, et al. Features of bilirubin-induced reactive microglia: from phagocytosis to inflammation. Neurobiol Dis 2010;40:663-75. [Crossref] [PubMed]
- Yueh MF, Chen S, Nguyen N, et al. Developmental onset of bilirubin-induced neurotoxicity involves Toll-like receptor 2-dependent signaling in humanized UDP-glucuronosyltransferase1 mice. J Biol Chem 2014;289:4699-709. [Crossref] [PubMed]
- Gomes-Leal W. Microglial physiopathology: how to explain the dual role of microglia after acute neural disorders? Brain Behav 2012;2:345-56. [Crossref] [PubMed]
- Bachiller S, Jimenez-Ferrer I, Paulus A, et al. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci 2018;12:488. [Crossref] [PubMed]
- Deczkowska A, Keren-Shaul H, Weiner A, et al. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell 2018;173:1073-81. [Crossref] [PubMed]
- Dubbelaar ML, Kracht L, Eggen BJL, et al. The kaleidoscope of microglial phenotypes. Front Immunol 2018;9:1753. [Crossref] [PubMed]
- Mosher KI, Wyss-Coray T. Microglial dysfunction in brain aging and Alzheimer's disease. Biochem Pharmacol 2014;88:594-604. [Crossref] [PubMed]
- Spittau B. Aging microglia-phenotypes, functions and implications for age-related neurodegenerative diseases. Front Aging Neurosci 2017;9:194. [Crossref] [PubMed]
- Olah M, Patrick E, Villani AC, et al. A transcriptomic atlas of aged human microglia. Nat Commun 2018;9:539. [Crossref] [PubMed]
- Caldeira C, Oliveira AF, Cunha C, et al. Microglia change from a reactive to an age-like phenotype with the time in culture. Front Cell Neurosci 2014;8:152. [Crossref] [PubMed]
- Caldeira C, Cunha C, Vaz AR, et al. Key aging-associated alterations in primary microglia response to beta-amyloid stimulation. Front Aging Neurosci 2017;9:277. [Crossref] [PubMed]
- Bachstetter AD, Van Eldik LJ, Schmitt FA, et al. Disease-related microglia heterogeneity in the hippocampus of Alzheimer's disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol Commun 2015;3:32. [Crossref] [PubMed]
- Walker DG, Lue LF. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther 2015;7:56. [Crossref] [PubMed]
- Rao Y, Liang YX, Peng B. A revisit of rod microglia in preclinical studies. Neural Regen Res 2017;12:56-7. [Crossref] [PubMed]
- Streit WJ, Xue QS, Tischer J, et al. Microglial pathology. Acta Neuropathol Commun 2014;2:142. [Crossref] [PubMed]
- Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of Alzheimer's disease. Cell 2017;169:1276-90.e17. [Crossref] [PubMed]
- Stratoulias V, Venero JL, Tremblay ME, et al. Microglial subtypes: diversity within the microglial community. EMBO J 2019;38:e101997. [Crossref] [PubMed]
- Butovsky O, Weiner HL. Microglial signatures and their role in health and disease. Nat Rev Neurosci 2018;19:622-35. [Crossref] [PubMed]
- Liaury K, Miyaoka T, Tsumori T, et al. Morphological features of microglial cells in the hippocampal dentate gyrus of Gunn rat: a possible schizophrenia animal model. J Neuroinflammation 2012;9:56. [Crossref] [PubMed]
- Vodret S, Bortolussi G, Jasprova J, et al. Inflammatory signature of cerebellar neurodegeneration during neonatal hyperbilirubinemia in Ugt1 (-/-) mouse model. J Neuroinflammation 2017;14:64. [Crossref] [PubMed]
- Masuda T, Sankowski R, Staszewski O, et al. Microglia heterogeneity in the single-cell era. Cell Rep 2020;30:1271-81. [Crossref] [PubMed]
- Angelova DM, Brown DR. Microglia and the aging brain: are senescent microglia the key to neurodegeneration? J Neurochem 2019;151:676-88. [Crossref] [PubMed]
- Streit WJ. Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci 2006;29:506-10. [Crossref] [PubMed]
- Vaz AR, Falcão AS, Scarpa E, et al. Microglia susceptibility to free bilirubin is age-dependent. Front Pharmacol 2020;11:1012. [Crossref] [PubMed]
- Brites D. Regulatory function of microRNAs in microglia. Glia 2020;68:1631-42. [Crossref] [PubMed]
- Carson MJ, Thrash JC, Walter B. The cellular response in neuroinflammation: the role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin Neurosci Res 2006;6:237-45. [Crossref] [PubMed]
- Sofroniew MV. Astrocyte reactivity: subtypes, states, and functions in cns innate immunity. Trends Immunol 2020;41:758-70. [Crossref] [PubMed]
- Escartin C, Galea E, Lakatos A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 2021;24:312-25. [Crossref] [PubMed]
- Amit Y, Brenner T. Age-dependent sensitivity of cultured rat glial cells to bilirubin toxicity. Exp Neurol 1993;121:248-55. [Crossref] [PubMed]
- Falcão AS, Silva RF, Pancadas S, et al. Apoptosis and impairment of neurite network by short exposure of immature rat cortical neurons to unconjugated bilirubin increase with cell differentiation and are additionally enhanced by an inflammatory stimulus. J Neurosci Res 2007;85:1229-39. [Crossref] [PubMed]
- Rhine WD, Schmitter SP, Yu AC, et al. Bilirubin toxicity and differentiation of cultured astrocytes. J Perinatol 1999;19:206-11. [Crossref] [PubMed]
- Chuniaud L, Dessante M, Chantoux F, et al. Cytotoxicity of bilirubin for human fibroblasts and rat astrocytes in culture. Effect of the ratio of bilirubin to serum albumin. Clin Chim Acta 1996;256:103-14. [Crossref] [PubMed]
- Siracusa R, Fusco R, Cuzzocrea S. Astrocytes: role and functions in brain pathologies. Front Pharmacol 2019;10:1114. [Crossref] [PubMed]
- Mahmoud S, Gharagozloo M, Simard C, et al. Astrocytes maintain glutamate homeostasis in the cns by controlling the balance between glutamate uptake and release. Cells 2019;8:184. [Crossref] [PubMed]
- Silva R, Mata LR, Gulbenkian S, et al. Inhibition of glutamate uptake by unconjugated bilirubin in cultured cortical rat astrocytes: role of concentration and pH. Biochem Biophys Res Commun 1999;265:67-72. [Crossref] [PubMed]
- Silva RF, Mata LM, Gulbenkian S, et al. Endocytosis in rat cultured astrocytes is inhibited by unconjugated bilirubin. Neurochem Res 2001;26:793-800. [Crossref] [PubMed]
- Brito MA, Rosa AI, Falcão AS, et al. Unconjugated bilirubin differentially affects the redox status of neuronal and astroglial cells. Neurobiol Dis 2008;29:30-40. [Crossref] [PubMed]
- Gennuso F, Fernetti C, Tirolo C, et al. Bilirubin protects astrocytes from its own toxicity by inducing up-regulation and translocation of multidrug resistance-associated protein 1 (Mrp1). Proc Natl Acad Sci USA 2004;101:2470-5. [Crossref] [PubMed]
- Garber C, Vasek MJ, Vollmer LL, et al. Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nat Immunol 2018;19:151-61. [Crossref] [PubMed]
- Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017;541:481-7. [Crossref] [PubMed]
- Röhl C, Lucius R, Sievers J. The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res 2007;1129:43-52. [Crossref] [PubMed]
- Kalmár B, Kittel A, Lemmens R, et al. Cultured astrocytes react to LPS with increased cyclooxygenase activity and phagocytosis. Neurochem Int 2001;38:453-61. [Crossref] [PubMed]
- Fernandes A, Silva RF, Falcão AS, et al. Cytokine production, glutamate release and cell death in rat cultured astrocytes treated with unconjugated bilirubin and LPS. J Neuroimmunol 2004;153:64-75. [Crossref] [PubMed]
- Kumral A, Genc S, Genc K, et al. Hyperbilirubinemic serum is cytotoxic and induces apoptosis in murine astrocytes. Biol Neonate 2005;87:99-104. [Crossref] [PubMed]
- Fernandes A, Falcão AS, Silva RF, et al. MAPKs are key players in mediating cytokine release and cell death induced by unconjugated bilirubin in cultured rat cortical astrocytes. Eur J Neurosci 2007;25:1058-68. [Crossref] [PubMed]
- Fernandes A, Falcão AS, Silva RF, et al. Inflammatory signalling pathways involved in astroglial activation by unconjugated bilirubin. J Neurochem 2006;96:1667-79. [Crossref] [PubMed]
- Fernandes A, Barateiro A, Falcão AS, et al. Astrocyte reactivity to unconjugated bilirubin requires TNF-alpha and IL-1beta receptor signaling pathways. Glia 2011;59:14-25. [Crossref] [PubMed]
- Fernandes A, Vaz AR, Falcão AS, et al. Glycoursodeoxycholic acid and interleukin-10 modulate the reactivity of rat cortical astrocytes to unconjugated bilirubin. J Neuropathol Exp Neurol 2007;66:789-98. [Crossref] [PubMed]
- Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev 2011;243:206-14. [Crossref] [PubMed]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009;7:99-109. [Crossref] [PubMed]
- Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 2017;277:61-75. [Crossref] [PubMed]
- Feng J, Li M, Wei Q, et al. Unconjugated bilirubin induces pyroptosis in cultured rat cortical astrocytes. J Neuroinflammation 2018;15:23. [Crossref] [PubMed]
- Gayen M, Bhomia M, Balakathiresan N, et al. Exosomal microRNAs released by activated astrocytes as potential neuroinflammatory biomarkers. Int J Mol Sci 2020;21:2312. [Crossref] [PubMed]
- Spampinato SF, Bortolotto V, Canonico PL, et al. Astrocyte-derived paracrine signals: relevance for neurogenic niche regulation and blood-brain barrier integrity. Front Pharmacol 2019;10:1346. [Crossref] [PubMed]
- Gomes C, Sequeira C, Barbosa M, et al. Astrocyte regional diversity in ALS includes distinct aberrant phenotypes with common and causal pathological processes. Exp Cell Res 2020;395:112209. [Crossref] [PubMed]
- Gomes C, Cunha C, Nascimento F, et al. Cortical neurotoxic astrocytes with early als pathology and mir-146a deficit replicate gliosis markers of symptomatic SOD1G93A mouse model. Mol Neurobiol 2019;56:2137-58. [Crossref] [PubMed]
- Laming PR, Kimelberg H, Robinson S, et al. Neuronal-glial interactions and behaviour. Neurosci Biobehav Rev 2000;24:295-340. [Crossref] [PubMed]
- Brites D, Fernandes A. Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci 2015;9:476. [Crossref] [PubMed]
- Brites D. Cell ageing: a flourishing field for neurodegenerative diseases. AIMS Molecular Science 2015;2:225-58. [Crossref]
- Doğaner BA, Yan LKQ, Youk H. Autocrine signaling and quorum sensing: extreme ends of a common spectrum. Trends Cell Biol 2016;26:262-71. [Crossref] [PubMed]
- Palmela I, Cardoso FL, Bernas M, et al. Elevated levels of bilirubin and long-term exposure impair human brain microvascular endothelial cell integrity. Curr Neurovasc Res 2011;8:153-69. [Crossref] [PubMed]
- Gazzin S, Berengeno AL, Strazielle N, et al. Modulation of Mrp1 (ABCc1) and Pgp (ABCb1) by bilirubin at the blood-CSF and blood-brain barriers in the Gunn rat. PLoS One 2011;6:e16165. [Crossref] [PubMed]
- Palmela I, Correia L, Silva RF, et al. Hydrophilic bile acids protect human blood-brain barrier endothelial cells from disruption by unconjugated bilirubin: an in vitro study. Front Neurosci 2015;9:80. [Crossref] [PubMed]
- Ives NK, Bolas NM, Gardiner RM. The effects of bilirubin on brain energy metabolism during hyperosmolar opening of the blood-brain barrier: an in vivo study using 31P nuclear magnetic resonance spectroscopy. Pediatr Res 1989;26:356-61. [Crossref] [PubMed]
- Wennberg RP. The blood-brain barrier and bilirubin encephalopathy. Cell Mol Neurobiol 2000;20:97-109. [Crossref] [PubMed]
- Lee C, Oh W, Stonestreet BS, et al. Permeability of the blood brain barrier for 125I-albumin-bound bilirubin in newborn piglets. Pediatr Res 1989;25:452-6. [Crossref] [PubMed]
- Bratlid D. How bilirubin gets into the brain. Clin Perinatol 1990;17:449-65. [Crossref] [PubMed]
- Wilton DK, Dissing-Olesen L, Stevens B. Neuron-glia signaling in synapse elimination. Annu Rev Neurosci 2019;42:107-27. [Crossref] [PubMed]
- Jang M, Gould E, Xu J, et al. Oligodendrocytes regulate presynaptic properties and neurotransmission through BDNF signaling in the mouse brainstem. Elife 2019;8:e42156. [Crossref] [PubMed]
- Bar El Y, Kanner S, Barzilai A, et al. Activity changes in neuron-astrocyte networks in culture under the effect of norepinephrine. PLoS One 2018;13:e0203761. [Crossref] [PubMed]
- Molofsky AV, Krencik R, Ullian EM, et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 2012;26:891-907. [Crossref] [PubMed]
- Hu W, Cheng X, Ye X, et al. Ex vivo (1)H nuclear magnetic resonance spectroscopy reveals systematic alterations in cerebral metabolites as the key pathogenetic mechanism of bilirubin encephalopathy. Mol Brain 2014;7:87. [Crossref] [PubMed]
- Linnerbauer M, Rothhammer V. Protective functions of reactive astrocytes following central nervous system insult. Front Immunol 2020;11:573256. [Crossref] [PubMed]
- Falcão AS, Silva RF, Vaz AR, et al. Cross-talk between neurons and astrocytes in response to bilirubin: early beneficial effects. Neurochem Res 2013;38:644-59. [Crossref] [PubMed]
- Falcão AS, Silva RF, Vaz AR, et al. Cross-talk between neurons and astrocytes in response to bilirubin: adverse secondary impacts. Neurotox Res 2014;26:1-15. [Crossref] [PubMed]
- Ferrini F, De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast 2013;2013:429815. [Crossref] [PubMed]
- Szepesi Z, Manouchehrian O, Bachiller S, et al. Bidirectional microglia-neuron communication in health and disease. Front Cell Neurosci 2018;12:323. [Crossref] [PubMed]
- Yong HYF, Rawji KS, Ghorbani S, et al. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol Immunol 2019;16:540-6. [Crossref] [PubMed]
- Sankowski R, Mader S, Valdes-Ferrer SI. Systemic inflammation and the brain: novel roles of genetic, molecular, and environmental cues as drivers of neurodegeneration. Front Cell Neurosci 2015;9:28. [Crossref] [PubMed]
- Simpson DSA, Oliver PL. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants (Basel) 2020;9:743. [Crossref] [PubMed]
- von Bernhardi R, Eugenin-von Bernhardi L, Eugenin J. Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci 2015;7:124. [Crossref] [PubMed]
- Zou J, Crews FT. Glutamate/NMDA excitotoxicity and HMGB1/TLR4 neuroimmune toxicity converge as components of neurodegeneration. Aims Molecular Science 2015;2:77-100. [Crossref]
- Porro C, Cianciulli A, Panaro MA. The regulatory role of IL-10 in neurodegenerative diseases. Biomolecules 2020;10:1017. [Crossref] [PubMed]
- Mishra MK, Rawji KS, Keough MB, et al. Harnessing the benefits of neuroinflammation:Generation of macrophages/microglia with prominent remyelinating properties. J Neurosci 2021;41:3366-85. [Crossref] [PubMed]
- Silva SL, Osorio C, Vaz AR, et al. Dynamics of neuron-glia interplay upon exposure to unconjugated bilirubin. J Neurochem 2011;117:412-24. [Crossref] [PubMed]
- Dani C, Pratesi S, Ilari A, et al. Neurotoxicity of unconjugated bilirubin in mature and immature rat organotypic hippocampal slice cultures. Neonatology 2019;115:217-25. [Crossref] [PubMed]
- Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol 2017;27:172-88. [Crossref] [PubMed]
- Hornung S, Dutta S, Bitan G. CNS-derived blood exosomes as a promising source of biomarkers: opportunities and challenges. Front Mol Neurosci 2020;13:38. [Crossref] [PubMed]
- Schulz J, Takousis P, Wohlers I, et al. Meta-analyses identify differentially expressed micrornas in Parkinson's disease. Ann Neurol 2019;85:835-51. [Crossref] [PubMed]
- Takousis P, Sadlon A, Schulz J, et al. Differential expression of microRNAs in Alzheimer's disease brain, blood, and cerebrospinal fluid. Alzheimers Dement 2019;15:1468-77. [Crossref] [PubMed]
- Burgos K, Malenica I, Metpally R, et al. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer's and Parkinson's diseases correlate with disease status and features of pathology. Plos One 2014;9:e94839. [Crossref] [PubMed]
- Spaull R, McPherson B, Gialeli A, et al. Exosomes populate the cerebrospinal fluid of preterm infants with post-haemorrhagic hydrocephalus. Int J Dev Neurosci 2019;73:59-65. [Crossref] [PubMed]
- Brito MA, Lima S, Fernandes A, et al. Bilirubin injury to neurons: contribution of oxidative stress and rescue by glycoursodeoxycholic acid. Neurotoxicology 2008;29:259-69. [Crossref] [PubMed]
- Akefi R, Hashemi SM, Alinejad S, et al. The effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy: a randomized clinical trial. J Matern Fetal Neonatal Med 2020;1-6. [Crossref] [PubMed]
- Gharehbaghi MM, Sani AM, Refeey M. Evaluating the effects of different doses of ursodeoxycholic acid on neonatal jaundice. Turk J Pediatr 2020;62:424-30. [Crossref] [PubMed]
- Liaury K, Miyaoka T, Tsumori T, et al. Minocycline improves recognition memory and attenuates microglial activation in Gunn rat: a possible hyperbilirubinemia-induced animal model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2014;50:184-90. [Crossref] [PubMed]
- Vodret S, Bortolussi G, Iaconcig A, et al. Attenuation of neuro-inflammation improves survival and neurodegeneration in a mouse model of severe neonatal hyperbilirubinemia. Brain Behav Immun 2018;70:166-78. [Crossref] [PubMed]
- Shi S, Cui Q, Xu J, et al. Baicalin suppresses bilirubin-induced apoptosis and inflammation by regulating p38 mitogen-activated protein kinases (MAPK) signaling in neonatal neurons. Med Sci Monit 2020;26:e926441. [Crossref] [PubMed]
- Boyanapalli SS, Tony Kong AN. "Curcumin, the King of Spices": epigenetic regulatory mechanisms in the prevention of cancer, neurological, and inflammatory diseases. Curr Pharmacol Rep 2015;1:129-39. [Crossref] [PubMed]
- Gazzin S, Dal Ben M, Montrone M, et al. Curcumin prevents cerebellar hypoplasia and restores the behavior in hyperbilirubinemic gunn rat by a pleiotropic effect on the molecular effectors of brain damage. Int J Mol Sci 2020;22:299. [Crossref] [PubMed]
- Cunningham AD, Hwang S, Mochly-Rosen D. Glucose-6-phosphate dehydrogenase deficiency and the need for a novel treatment to prevent kernicterus. Clin Perinatol 2016;43:341-54. [Crossref] [PubMed]
- Deliktaş M, Ergin H, Demiray A, et al. Caffeine prevents bilirubin-induced cytotoxicity in cultured newborn rat astrocytes. J Matern Fetal Neonatal Med 2019;32:1813-9. [Crossref] [PubMed]
- Brubaker DK, Lauffenburger DA. Translating preclinical models to humans. Science 2020;367:742-3. [Crossref] [PubMed]
- Fumagalli M, Lombardi M, Gressens P, et al. How to reprogram microglia toward beneficial functions. Glia 2018;66:2531-49. [Crossref] [PubMed]
- Chen Y, Li YF, Wang X, et al. Amelioration of hyperbilirubinemia in Gunn rats after transplantation of human induced pluripotent stem cell-derived hepatocytes. Stem Cell Reports 2015;5:22-30. [Crossref] [PubMed]
- Fu SY, Wang Y, Xia XH, et al. Exosome engineering: current progress in cargo loading and targeted delivery. Nanoimpact 2020;20:100261. [Crossref]
- Villarreal-Leal RA, Cooke John P, Bruna C. Biomimetic and immunomodulatory therapeutics as an alternative to natural exosomes for vascular and cardiac applications. Nanomedicine 2021;102385. [Crossref] [PubMed]
- Munir J, Yoon JK, Ryu S. Therapeutic miRNA-enriched extracellular vesicles: current approaches and future prospects. Cells 2020;9:2271. [Crossref] [PubMed]
Cite this article as: Brites D, Silva RFM. Bilirubin neurotoxicity: a narrative review on long lasting, insidious, and dangerous effects. Pediatr Med 2021;4:34.

