
Significance of red blood cell distribution width in children with celiac disease
Introduction
Celiac disease (CD) is an immune-mediated inflammatory disease of the small bowel secondary to dietary gluten sensitivity in individuals with genetic predisposition. Signs and symptoms vary greatly and range from intestinal symptoms such as diarrhea, abdominal pain or distension, weight loss, and failure to thrive to extraintestinal symptoms such as anemia, osteopenia, and short stature to name a few. However, in a multi-institutional study, prevalence of CD in the United States was noted to be 1:133 among patients with no risk factors or symptoms (1).
As per North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) guidelines, the standard screening test for CD is Tissue Transglutaminase immunoglobulin A (tTG-IgA). Total IgA is additionally obtained to determine if patient has an underlying deficiency. In some clinical scenarios, additional celiac serology such as the Anti-Endomysial IgA or the Deamidated Gliadin Peptide is obtained. Diagnosis is confirmed by esophagogastroduodenoscopy. As per NASPHGAN guidelines, 1
Standard management of patients diagnosed with CD consists of gluten-free diet (GFD) (3). Subsequent antibody testing is often performed 4-6 months after treatment has begun. In addition to monitoring growth parameters, other routine laboratory tests, while not universally performed, can include complete blood count (CBC), thyroid stimulating hormone, comprehensive metabolic panel, iron profile, vitamin B12, vitamin D, and folate.
Iron deficiency anemia can be a known complication or initial presentation of CD (4). Ferritin is commonly monitored to screen for iron deficiency anemia in patients with CD as well. With ferritin being an inflammatory marker, it can be falsely negative in patients with inflammatory conditions (5), such as CD. Red blood cell distribution width (RDW) may be a more accurate marker to assess for iron deficiency in patients with CD (6). RDW is the measurement of heterogeneity of red blood cells as well as size variation in peripheral blood, or anisocytosis (7). It is a typically included parameter of a CBC, which is widely available and inexpensive to obtain (6). The value of RDW in assessing clinical outcomes and disease severity has been shown across multiple medical conditions, such as being elevated in patients with iron deficiency anemia (8). Mortality and hospital survival rate assessment have also been shown to be a utility of RDW (9).
In a study of 126 adult patients with CD, Brusco et al. found increased RDW to be the most frequent abnormality from a hematologic standpoint (58%) with the next most frequent being anemia (31%) and iron deficiency (29%) (10). Another retrospective study by Harmanci et al. comparing RDW values according to presence of intestinal atrophy concluded RDW value >17.25% to be a significant predictor of atrophy (P=0.003) (11). This study included 49 newly diagnosed CD patients. Additionally, RDW was also used to assess response to GFD (11,12). As Mitchell et al. showed a significant decrease from initial elevated levels (17.3% vs. 13.8%) after 12 months of GFD diet in adult CD patients (12).
Due to increased incidence and prevalence of CD, there is potential utility of simple, inexpensive laboratory tests for diagnosing and monitoring adherence to GFD, especially if additional laboratory workup is equivocal. Our study aims to determine if RDW is a reliable marker of compliance in children treated for CD, like that of the adult counterpart studies. Additionally, we aim to determine if RDW is a reliable prognostic factor in severity of CD in children. We hypothesize that RDW is a useful marker of compliance in children treated for CD and that RDW is a potential predictor of intestinal atrophy in the pediatric CD population. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/pm-21-24).
Methods
This study consisted of a retrospective chart review of all children ages 1
Patients were excluded if there was no documented CBC with reviewable lab values prior to diagnosis or if the patient already practiced a gluten free diet. Additional exclusions consisted of the following: patients less than 1 or greater than 17 years of age, documented history of repetitive noncompliance to previous diets recommended by physician, presence of comorbid conditions potentially affecting CBC (i.e., hematologic, oncologic, cardiovascular, renal, inflammatory bowel disease, hepatic), and patients on treatment for previously known anemia. This study was approved by the Wayne State Institutional Review Board as well as Detroit Medical Center Institutional Review Board (IRB# 045118MP4E, protocol # 1804001374). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Statistical analysis
Statistical analysis of data was performed using SPSS (Statistical Package for the Social Sciences) for Windows Release 24.0 (SPSS Inc., Chicago, IL, USA). The primary outcome consisting of mean difference in RDW pre- and post-GFD was examined using an independent sample t-test. If assumptions of normality and homogeneity of variance were violated, a non-parametric Mann Whitney U test was substituted. A non-parametric Spearman Rho test examined whether a correlation exists between mean difference in RDW and mean difference in tTG-IgA. Exploratory secondary outcomes consisted of analyzing RDW values and determining if a cut-off level led to a significant difference between the presence or absence of atrophy. If assumptions were violated, a non-parametric Kruskal Wallis test was substituted, and pair-wise comparisons were conducted using Mann Whitney U tests. Any comparisons between proportions were examined using a non-parametric Fisher’s Exact Test. Significant differences were considered achieved at a P value less than 0.05.
Results
Demographics
A total of 336 patients were in our study cohort. Among them, 128 patients met inclusion criteria. And 83/128 (65%) were female. Racial demographics: 105/128 (82%) Caucasian, 4/128 (3%) African American, 3/128 (2%) Hispanic/Latino, and 16/128 (13%) races were designated other or unknown. Ages ranged from 1
Serology
Mean initial RDW prior to GFD was 13.3±0.89, while follow-up RDW mean was 13.1±0.84 (reference range 11.7
Atrophy
The 79/128 (62%) of patient pathology reports demonstrated presence of atrophy at initial diagnosis. Mean initial RDW was 13.4±0.93 in patients with atrophy, compared to 13.2±0.80 in those patients with no atrophy; this was not found to be significant (P=0.113). Other labs reviewed in relation to villous atrophy are noted in Table 1. However, there was a statistically significant association among patients having an initial RDW >12.9 and presence of atrophy (Table 2). Pre-GFD, 63.3% of patients with absence of atrophy had RDW values >12.9 in comparison to 79.7% with atrophy. Further, there was also a statistically significant association among patients having a follow-up RDW >12.9 and no atrophy at time of diagnosis. Post-GFD, 36.7% of patients with absence of atrophy had RDW values >12.9 in comparison to 59.5% with atrophy.
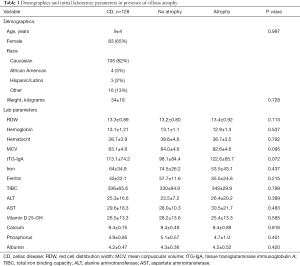
Full table

Full table
There was a significant difference in the tTG-IgA values obtained at the time of diagnosis between patients with and without villous atrophy (129.0 vs. 83.0 U/L, P=0.010). However, no significant median differences were found in follow-up tTG-IgA values between the no atrophy group and the atrophy group (10.0 vs. 21.0 U/L, P=0.370).
Discussion
Compliance
The relationship with iron deficiency anemia and CD has been well established. It has been shown that patients with CD can present with iron deficiency due to malabsorption. However, this relationship is poorly understood as patients without villous atrophy can also present with anemia. While anemia does not improve in all patients with dietary modification, it does in the majority. Compliance on a GFD therefore remains of utmost importance.
An iron profile and a CBC are often monitored to assess GFD compliance as well as iron deficiency anemia, with the expectations that they will normalize in patients that are compliant. This may not be accurate given that ferritin may be falsely elevated as it is an inflammatory marker (5). MCV is also potentially not reliable in CD since this condition can cause anemia of chronic disease (13,14). RDW may be a more accurate marker, which is inexpensive and easy to obtain (6). We therefore hypothesized that RDW value difference pre- and post-GFD may potentially be useful as a marker of compliance in the pediatric population. Compliance in our study was determined by clinical history and further supported by decrease in tTG-IgA. It should be noted that none of our patients were on iron supplementation, as they all responded to GFD. In our study, the decrease in mean RDW value from diagnosis to follow-up GFD was not found to be statistically significant (P=0.585). Brusco et al. found that RDW was the most prominent hematologic abnormality in an adult CD study of 126 patients (10). Our study of 128 patients, being unique to the pediatric population, did not show consistently abnormal values for RDW. Despite our findings there does appear to be an indirect association given the mean decrease noted. There have been multiple adult studies demonstrating an improvement in RDW after following a GFD (6,11,12). The argument may be made that lab values in the pediatric CD population may not reflect the same level of chronicity as comparatively longstanding disease in adults.
Atrophy
The classic intestinal manifestation of CD seen on small intestinal biopsy is villous atrophy. In the pediatric CD population, atrophy becomes especially relevant due to the impact on nutrition and achievement of maximum growth potential. Our study showed that at diagnosis there was no statistical difference in the mean RDW with or without atrophy (P=0.113). However, further analysis determined that there was a statistically significant association among patients having a RDW >12.9 and presence of atrophy (P=0.04).
This potentially supports the utility of close monitoring of RDW as a predictive marker of atrophy and by extension CD in the appropriate clinical setting. RDW has been suggested to serve as an early sign of intestinal disease even prior to other hematologic parameters. This was noted in a 2002 prospective adult study where there was an increase noted in RDW value in patients with CD when compared to normal individuals, despite normal hemoglobin values (15). Furthermore, in a 2017 prospective pediatric study there were 19 patients defined as having potential or latent CD (positive CD screening but no mucosal atrophy) (16). Knowing that this cohort of patients were either demonstrating signs of CD prior to mucosal changes or would eventually develop mucosal changes, an iron panel was obtained. Among these patients, 0/19 (0%) had low iron, 4/19 (21%) had low ferritin, 3/19 (15%) had anemia. RDW was not mentioned in this study, but we suspect that a larger proportion of patients in this cohort had abnormal RDW. This is suggested by RDW being a more sensitive marker in patients with inflammatory conditions as previously stated. This is our hypothesis and would require further confirmation with additional studies.
It is plausible that early manifestations such as laboratory values in the appropriate clinical setting may lead to earlier CD screening, diagnosis, and treatment. In a climate of strong patient interest in empiric dietary modification, increased identification of latent CD, and high healthcare costs, the utility of routine low-cost testing may be effective in guiding further workup.
To our knowledge this is the first study in the pediatric population to investigate the utility of RDW specifically as a potential predictive marker of both compliance and intestinal atrophy in CD. The strengths of our study include the size of our patient population meeting inclusion criteria. This allowed for more direct comparison between previous adult studies with typically larger patient volumes.
Challenges faced with this study included limited documentation of other lab parameters of interest (i.e., B12, folate, vitamin D, Iron Panel) prior to diagnosis. This limited the ability to conduct more extensive analysis of these potentially relevant nutritional values. Additionally, future prospective studies would be needed to establish a more robust temporal relationship between RDW and atrophy.
In conclusion, RDW did not seem to be as sensitive as a marker for CD patients as compared to adult studies in either severity or compliance. This is an important finding, as the use of RDW is becoming more widespread due to its ease of use, economic feasibility, and significance in assessing a variety of pathologic conditions. Since this is the first pediatric study examining the relationship RDW and CD, more research is needed in this area. However, our data shows that there is disparity between adult studies and pediatric studies in the usefulness in RDW as a predictive marker for intestinal atrophy and compliance in patients with CD.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/pm-21-24
Data Sharing Statement: Available at https://dx.doi.org/10.21037/pm-21-24
Peer Review File: Available at https://dx.doi.org/10.21037/pm-21-24.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/pm-21-24). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Wayne State Institutional Review Board as well as Detroit Medical Center Institutional Review Board (IRB# 045118MP4E, protocol # 1804001374). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med 2003;163:286-92. [Crossref] [PubMed]
- Snyder J, Butzner JD, DeFelice AR, et al. Evidence-Informed Expert Recommendations for the Management of Celiac Disease in Children. Pediatrics 2016;138:e20153147 [Crossref] [PubMed]
- Guandalini S, Assiri A. Celiac disease: a review. JAMA Pediatr 2014;168:272-8. [Crossref] [PubMed]
- Halfdanarson TR, Litzow MR, Murray JA. Hematologic manifestations of celiac disease. Blood 2007;109:412-21. [Crossref] [PubMed]
- Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014;6:748-73. [Crossref] [PubMed]
- Goyal H, Lippi G, Gjymishka A, et al. Prognostic significance of red blood cell distribution width in gastrointestinal disorders. World J Gastroenterol 2017;23:4879-91. [Crossref] [PubMed]
- Daland GA, Heath CW, Minot GR. Differentiation of pernicious anemia and certain other macrocytic anemias by the distribution of red blood cell diameters. Blood 1946;1:67-75. [Crossref] [PubMed]
- Sazawal S, Dhingra U, Dhingra P, et al. Efficiency of red cell distribution width in identification of children aged 1-3 years with iron deficiency anemia against traditional hematological markers. BMC Pediatr 2014;14:8. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C, Cervellin G. Learning more and spending less with neglected laboratory parameters: the paradigmatic case of red blood cell distribution width. Acta Biomed 2016;87:323-8. [PubMed]
- Brusco G, Di Stefano M, Corazza GR. Increased red cell distribution width and coeliac disease. Dig Liver Dis 2000;32:128-30. [Crossref] [PubMed]
- Harmanci O, Kav T, Sivri B. Red cell distribution width can predict intestinal atrophy in selected patients with celiac disease. J Clin Lab Anal 2012;26:497-502. [Crossref] [PubMed]
- Mitchell RM, Robinson TJ. Monitoring dietary compliance in coeliac disease using red cell distribution width. Int J Clin Pract 2002;56:249-50. [PubMed]
- Harper JW, Holleran SF, Ramakrishnan R, et al. Anemia in celiac disease is multifactorial in etiology. Am J Hematol 2007;82:996-1000. [Crossref] [PubMed]
- Bergamaschi G, Markopoulos K, Albertini R, et al. Anemia of chronic disease and defective erythropoietin production in patients with celiac disease. Haematologica 2008;93:1785-91. [Crossref] [PubMed]
- Sategna Guidetti C, Scaglione N, Martini S. Red cell distribution width as a marker of coeliac disease: a prospective study. Eur J Gastroenterol Hepatol 2002;14:177-81. [Crossref] [PubMed]
- Repo M, Lindfors K, Mäki M, et al. Anemia and Iron Deficiency in Children With Potential Celiac Disease. J Pediatr Gastroenterol Nutr 2017;64:56-62. [Crossref] [PubMed]
Cite this article as: Cortes O, Rabbani T, Thomas R, Cares K. Significance of red blood cell distribution width in children with celiac disease. Pediatr Med 2021;4:25.

