Management of rapidly growing recurrent extra-abdominal pediatric desmoid tumor: case report
Introduction
Desmoid tumors are rare, soft tissue tumors that comprise <3% of soft tissue malignancies (1). Management of these locally aggressive tumors can involve multiple modes of therapy including surgical resection, radiotherapy, and systemic therapy. However, even after seemingly adequate treatment, recurrence rates remain high. The recurrent nature of these tumors lends them difficult to manage and may result in a significant source of patient morbidity and mortality. We discuss a patient with a genetic predisposition for desmoid tumors who initially presented at an early age, and developing recurrent disease requiring multiple treatment regimens given the unrelenting and unpredictable nature of the disease. We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/pm-21-28).
Case presentation
We present a case of a 9-year-old African American male with Gardner syndrome and a large recurrent desmoid tumor on his back. The patient first presented to us at age 4 years, for a new small desmoid tumor on the sternum. Remote history revealed that small tumors had been visible on his back by six months of age. At 15 months of age, he had begun to undergo multiple surgeries by both orthopedic and plastic surgery. Pathologically the lesions were consistent with aggressive fibromatosis, with and without positive margins. Genetics evaluation had identified a heterozygous deletion mutation in exon 15 of the APC gene, confirming the diagnosis of familial adenosis polyposis (FAP) or Gardner syndrome.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the Helsinki Declaration (as revised in 2013). The study was approved by the submitting research institute, neither approval from the ethics committee nor informed consent from the study population was required given the number of cases being presented. No patient identifiers are included in the text and no facial features are present in the images.
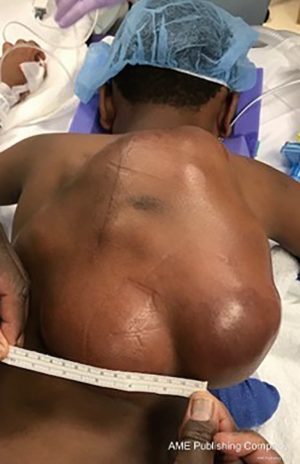
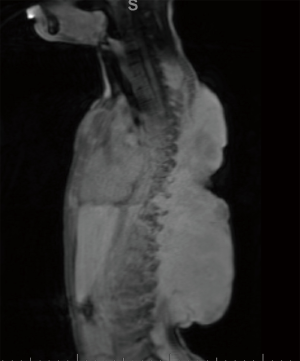
At first evaluation, the patient had several palpable asymptomatic tumors about the scalp, ribs, back, and sternum, the largest of which was ~4 cm. Observation was recommended. The patient was lost to follow up for one year, and at his return, a dominant lesion on the posterior trunk had shown substantial interval growth (largest dimension ~11 cm) with accompanying pain, whereas other lesions on the scalp, ribs, and sternum remained stable or regressed. Initial therapy with oral imatinib was begun. Four months later, MRI confirmed interval growth of the largest posterior lesion with largest dimension measuring over 16cm. Therapy was changed to methotrexate and vinblastine. Again, after four months, the dominant posterior tumor showed interval growth and therapy was changed to doxorubicin monotherapy (six cycles), which provided radiographic stability for approximately eight months before again drastic tumor progression was noted. At this time, MRI demonstrated the largest mass measuring 20×6.5×18.4 cm3 with the involvement of the vertebral lamina and transverse processes with extension into the spinal canal at T10–T11 without spinal cord involvement (Figure 1).
Due to worsening pain, significant cosmetic effect on the patient, and failure of several systemic therapies, a multidisciplinary tumor board recommended proceeding with surgical debulking of the mass followed by adjuvant proton-beam radiotherapy. Intraoperatively, the tumor measured 30×25 cm2 (Figure 2), with approximately 50% debulked. Pathologic examination of the tumor was consistent with aggressive fibromatosis. The proximity of the tumor to the dermis made leaving adequate skin flaps for primary closure very difficult. He remained inpatient for two weeks post-operatively for extensive wound care and serial dressing changes.
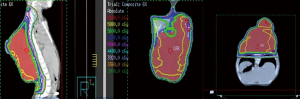
Proton-beam radiotherapy was initially planned 4 weeks post-operation; however, he developed necrosis of the skin flaps requiring placement of a split-thickness skin graft (14×8 cm2) to cover the exposed tumor bed, delaying radiotherapy by one-month. At the time of initiating radiotherapy, the tumor had grown to a size larger than its pre-debulking size (Figure 3). The patient was started on pencil beam-scanning proton therapy, but after receiving 4 CGE in two fractions, the tumor was noticeably larger. Due to the range uncertainty of proton beam therapy with changes in tumor size, treatment was changed to photon therapy. After the delivery of 24 Gy in 12 fractions, tumor growth persisted, prompting delivery of an additional 32 Gy. In total, the tumor received 60 Gy in 30 fractions. Figure 4 depicts a summary of the radiation fields and dose distribution. For target delineation, a gross tumor volume (GTV) was contoured including any visible tumor on all available imaging. The clinical target volume (CTV) included GTV with a 2-cm margin constrained by natural anatomic boundaries and a planned target volume (PTV) of 0.5-cm was used to account for potential set up errors. By the last week of treatment, the tumor started to decrease in size.
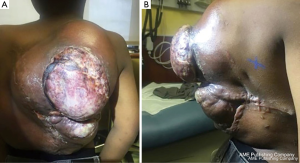
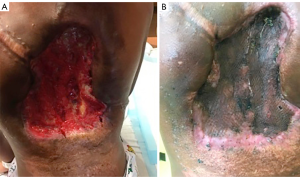
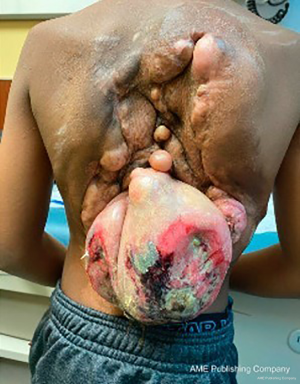
One week after completing radiotherapy, he developed foul-smelling, necrotic drainage from the tumor and wound site. He underwent serial debridement with removal of a significant amount of necrotic tumor with a >70% reduction in tumor size. He eventually had a split-thickness skin graft placed to cover the resection site and was started on pazopanib for maintenance therapy (Figure 5). The tumor remained stable after the dramatic response to radiotherapy, unfortunately it lasted merely six months, when evidence of tumor growth reappeared in the tumor bed. He was transitioned to sorafenib; however, the tumor continued its uncontrollable and dysmorphic growth (Figure 6), requiring re-excision 12 months later and consideration for new experimental trial therapies. As of this writing, the patient has shown disease progression on nirogacestat, a gamma secretase inhibitor, obtained through expanded access that is now being evaluated in an open clinical trial for patients <18 years, and he is receiving palliative bevacizumab and low dose weekly vincristine. Further surgical intervention has not been attempted due to bleeding risk and poor nutritional status.
Discussion
Aggressive fibromatosis, also known as desmoid tumors, are rare, benign, locally aggressive tumors with high recurrence rates, even after complete microscopic (R0) resection. They comprise 0.03% of all tumors and <3% of soft tissue tumors (1). The majority occur sporadically, although up to 15% occur in patients with familial adenomatous polyposis (FAP), also known as Gardner syndrome (2). The risk of developing desmoid tumors is much higher in the setting of FAP where they are a significant source of morbidity and mortality as they are commonly found to present earlier in life (compared to sporadic tumors), in males, and located intra-abdominal or within the abdominal wall (as opposed to extra-abdominal as typically seen in sporadic tumors), with a fondness for previous surgical sites (2).
The unpredictable nature of desmoid tumors makes them very challenging to treat and a source of significant morbidity and mortality. They can spontaneously regress, remain stable, or grow uncontrollably (3). For desmoid tumors that spontaneously regress or those that are asymptomatic and stable, observation or “wait-and-see” is the best option for therapy (4). A comparison of observation to hormonal therapy or chemotherapy demonstrated equivalent 5-year progression-free survival (49.9% for observation vs. 58.6% for hormonal/chemotherapy, P=0.32) (4).
Controversy arises when discussing the management of symptomatic or progressively enlarging desmoid tumors, as in our case. Treatment options include surgery alone, radiation alone, a combination of surgery and radiation, and systemic therapy. Traditional therapy for desmoid tumors involved an R0 resection. Complete tumor excision avoids subjecting pediatric patients to radiation or chemotherapy, which have long-term sequelae, like lymphangitis, pathologic fractures, fibrosis of adjacent structures, and secondary malignancies (5,6).
There is evidence to suggest surgery alone is not always the best therapy for desmoid tumors. A comparative review of 22 articles with a median follow-up between 2 to 10.4 years showed that radiation therapy (RT) or a combination of surgery and radiation (S+RT) achieved local control significantly better than surgery alone (S) (78% RT vs. 61% S, P=0.02 and 75% S+RT vs. 61% S, P=0.0002) (5). Additionally, local control was better achieved for negative and positive-surgical margins with the addition of adjuvant RT (negative margins: 94% S+RT vs. 72% S, P=0.004 and positive margins: 75% S+RT vs. 41% S, P<0.001) (5). Furthermore, for recurrent tumors after previous resection, local control was significantly improved with the addition of adjuvant RT (79% S+RT vs. 47% S, P<0.001) (5,6).
When comparing surgical resection with negative margins and RT, Ballo et al. reported similar 10-year relapse rate in their review of 189 cases (27% S vs. 25% RT) (7). Patients with positive surgical margins had significantly higher relapse rates compared to negative surgical margins (54% vs. 27%, P=0.003) (7). Similarly, in a review of 177 patients, Mullen et al. reported an improved 10-year recurrence-free survival with negative surgical margins (77% for negative-margins vs. 52% for positive-margins, P=0.006) with the only predictor of recurrence-free survival being a complete microscopic resection on multi-variable analysis (8). However, the emphasis on obtaining a complete microscopic resection has been questioned with multiple studies suggesting an R1 resection (positive microscopic margins) is not a negative prognostic indicator of recurrence (9-11). Furthermore, it has been suggested that surgical resection with negative margins is the treatment of choice when possible; however, resections should be structure and function-sparing with the use of adjuvant RT for positive margins (50–60 Gy for microscopic and 60–65 Gy for gross) (7,9-14). Given the massive nature of our patients’ tumor with involvement of the spine and having failed multiple cytotoxic therapies to help cease or control tumor growth, a structure and function-sparing debulking of the tumor was performed, followed by adjuvant RT. Despite a considerable initial response, it was short-lived as tumor growth resumed six months later.
Systemic therapies can be considered for patients with rapid locoregional recurrence despite adequate surgical resection, unresectable tumors that are symptomatic, or asymptomatic unresectable tumors that are rapidly progressing. Systemic therapies are divided into non-cytotoxic and cytotoxic therapies. Non-cytotoxic therapies include non-steroidal anti-inflammatory drugs (NSAIDs) (sulindac and celecoxib), anti-estrogens (tamoxifen), and tyrosine kinase inhibitors (imatinib, pazopanib, and sorafenib). Cytotoxic therapies include methotrexate and doxorubicin-based regimens.
Evaluating the success of non-cytotoxic therapies is difficult, given the slow growth of desmoid tumors and the often-delayed manifestation of treatment results. Studies have demonstrated <50% clinical improvement from NSAIDs, tamoxifen, and imatinib therapy, with one study showing no clinical benefit in patients with FAP (15,16). Pazopanib and sorafenib are generally preferred over imatinib because they have a higher degree of activity; however, they are more toxic. In a randomized phase III trial, 2-year progression-free survival in patients taking sorafenib was improved to 81%, compared to 36% in the placebo group (P<0.001) (17). Similarly, pazopanib demonstrated the potential to halt progression, possibly better than some cytotoxic regimens as exhibited in a randomized phase II trial (18).
Cytotoxic methotrexate-based regimens have demonstrated partial response with a decrease in tumor size or disease stabilization in 70–100% of patients with a median progression-free survival of 75 months (19,20). Doxorubicin-based regimens have demonstrated better control rates, with 93% of tumors shrinking or remaining stable in some studies (11,16). These control rates were maintained after 31 months of follow-up, which is favorable given 80% of disease recurrence or progression occurs within 3 years of treatment (15). Doxorubicin-based regimens, however, are more toxic compared to methotrexate-based chemotherapy with adverse reactions including mucositis, nausea, myelosuppression, and cardiac toxicity (19).
Due to the rarity and variability in the location and behavior of desmoid tumors, various therapy options exist without clear superiority of any treatment approach. Our patients’ best response was following RT, although progression-free survival was brief. When a complete function-sparing tumor resection is not possible or a patient has recurrent disease, adjuvant RT should be strongly considered early, as tumor cells that progress after multiple lines of systemic therapy may acquire resistance to radiation. Although not feasible in our case, proton-beam radiotherapy may be considered in the pediatric population to potentially limit the development of sequelae from therapy later on in life, particularly in patients with FAP, who will require life-long surveillance for several malignancies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/pm-21-28
Peer Review File: Available at https://dx.doi.org/10.21037/pm-21-28
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/pm-21-28). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the Helsinki Declaration (as revised in 2013). The study was approved by the submitting research institute, neither approval from the ethics committee nor informed consent from the study population was required given the number of cases being presented. No patient identifiers are included in the text and no facial features are present in the images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reitamo JJ, Häyry P, Nykyri E, et al. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. Am J Clin Pathol 1982;77:665-73. [Crossref] [PubMed]
- Nieuwenhuis MH, Casparie M, Mathus-Vliegen LM, et al. A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int J Cancer 2011;129:256-61. [Crossref] [PubMed]
- Bonvalot S, Ternès N, Fiore M, et al. Spontaneous regression of primary abdominal wall desmoid tumors: more common than previously thought. Ann Surg Oncol 2013;20:4096-102. [Crossref] [PubMed]
- Fiore M, Rimareix F, Mariani L, et al. Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol 2009;16:2587-93. [Crossref] [PubMed]
- Nuyttens JJ, Rust PF, Thomas CR Jr, et al. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: A comparative review of 22 articles. Cancer 2000;88:1517-23. [Crossref] [PubMed]
- Ergen ŞA, Tiken EE, Öksüz DÇ, et al. The Role of Radiotherapy in the Treatment of Primary or Recurrent Desmoid Tumors and Long-Term Results. Balkan Med J 2016;33:316-21. [Crossref] [PubMed]
- Ballo MT, Zagars GK, Pollack A, et al. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol 1999;17:158-67. [Crossref] [PubMed]
- Mullen JT, Delaney TF, Kobayashi WK, et al. Desmoid tumor: analysis of prognostic factors and outcomes in a surgical series. Ann Surg Oncol 2012;19:4028-35. [Crossref] [PubMed]
- Salas S, Dufresne A, Bui B, et al. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-and-see policy according to tumor presentation. J Clin Oncol 2011;29:3553-8. [Crossref] [PubMed]
- Merchant NB, Lewis JJ, Woodruff JM, et al. Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer 1999;86:2045-52. [Crossref] [PubMed]
- Lev D, Kotilingam D, Wei C, et al. Optimizing treatment of desmoid tumors. J Clin Oncol 2007;25:1785-91. [Crossref] [PubMed]
- Ballo MT, Zagars GK, Pollack A. Radiation therapy in the management of desmoid tumors. Int J Radiat Oncol Biol Phys 1998;42:1007-14. [Crossref] [PubMed]
- Janssen ML, van Broekhoven DL, Cates JM, et al. Meta-analysis of the influence of surgical margin and adjuvant radiotherapy on local recurrence after resection of sporadic desmoid-type fibromatosis. Br J Surg 2017;104:347-57. [Crossref] [PubMed]
- Spear MA, Jennings LC, Mankin HJ, et al. Individualizing management of aggressive fibromatoses. Int J Radiat Oncol Biol Phys 1998;40:637-45. [Crossref] [PubMed]
- Patel SR, Benjamin RS. Desmoid tumors respond to chemotherapy: defying the dogma in oncology. J Clin Oncol 2006;24:11-2. [Crossref] [PubMed]
- Bertagnolli MM, Morgan JA, Fletcher CD, et al. Multimodality treatment of mesenteric desmoid tumours. Eur J Cancer 2008;44:2404-10. [Crossref] [PubMed]
- Gounder MM, Mahoney MR, Van Tine BA, et al. Sorafenib for Advanced and Refractory Desmoid Tumors. N Engl J Med 2018;379:2417-28. [Crossref] [PubMed]
- Toulmonde M, Pulido M, Ray-Coquard I, et al. Pazopanib or methotrexate-vinblastine combination chemotherapy in adult patients with progressive desmoid tumours (DESMOPAZ): a non-comparative, randomised, open-label, multicentre, phase 2 study. Lancet Oncol 2019;20:1263-72. [Crossref] [PubMed]
- Constantinidou A, Jones RL, Scurr M, et al. Advanced aggressive fibromatosis: Effective palliation with chemotherapy. Acta Oncol 2011;50:455-61. [Crossref] [PubMed]
- Palassini E, Frezza AM, Mariani L, et al. Long-term Efficacy of Methotrexate Plus Vinblastine/Vinorelbine in a Large Series of Patients Affected by Desmoid-Type Fibromatosis. Cancer J 2017;23:86-91. [Crossref] [PubMed]
Cite this article as: Al-Hadidi A, Almahariq MF, Osei SK, Gowans LK, Stallion A. Management of rapidly growing recurrent extra-abdominal pediatric desmoid tumor: case report. Pediatr Med 2021;4:30.