
Current outcomes and future trends in paediatric and congenital cardiac surgery: a narrative review
Introduction
Congenital heart disease (CHD) comprises a colorful spectrum of anomalies resulting from abnormal development of the heart segments and the great arteries during fetal life. CHD is the most common birth defect and its incidence ranges from 6 to 13 per 1,000 live births affecting approximately 1 neonate in every 120–166 births (1,2). It is estimated that about 1,310,000 babies are born with CHD worldwide each year (3). CHD prevalence in the population has grossly increased as survival to adulthood now reaches 90–95% in high-income countries from less than 20% in the presurgical era (4). In the 2020s, the number of grown-ups living with CHD is expected to surpass the neonatal/infant CHD incidence (5,6). About 15% of CHD patients who previously underwent cardiac surgery will require subsequent re-operations (e.g., conduit exchange, valve repairs/replacements, operation for acquired hearts disease, etc.). Treating patients with CHD has a significant public health aspect and it is a commitment for life (7).
Congenital cardiac anomalies present along the full spectrum in the connections of the cardiac segments, their morphology, their relations, and additional anomalies in any segment (8). About 52–66% percent of congenital cardiac patients need to undergo cardiac surgery in their lifetime (1,9); and half of the surgical patient population requires intervention within the first six months of life (1). Primary complete repair has become a central aim of surgery since the 1980s, where all intracardiac and extracardiac issues would be dealt with by a single-stage operation (10). Surgical repair may nowadays restore adequate intracardiac and extracardiac segmental connections in the minimum range of 0.1 mm, however it has so far been unable to address underlying pathologic/developmental processes that occur in the range of nanometers to micrometers.
In about thirty percent of all congenital cardiac surgical cases, anomalies cannot be solved by a single operation (11,12). Physiologic and anatomical reasons, e.g., state of the pulmonary vasculature perfused by major aortopulmonary collaterals (MAPCAs) or hypoplasia may necessitate staged preparatory operative steps. Implanted valves and/or conduits disintegrate or are outgrown with time, rendering the recipients to reoperations. Congenital cardiac surgery is reconstructive surgery with the aim of restoring biventricular circulation when possible. In the absence of two functionally adequate pumping chamber (ventricle), for the presently lacking clinically applicable regeneration methods (13), univentricular rerouting (Fontan-circulation) remains the only surgical solution (14-16). Specifics of the univentricular physiology do not currently permit neonatal establishment of the Fontan-circulation, so these patients should also undergo staged-repairs.
Contemporary mortality rate of congenital cardiac surgery for neonates, infants, and children is reported between lower limit of 0.1% (repair of subvalvar aortic stenosis) and upper limit of 13.2% (Norwood-procedure) (17) that places this human endeavor in the highest risk-range (18). Especially, neonates undergoing complex open-heart procedures in high acuity are at high short-term risk. Long-term risk derives from the delay in somatomental growth and is related to ongoing pathophysiological processes. As premised, congenital cardiac surgery is mainly reconstructive in its nature and applies individual solutions for the wide variety of defects.
Current surgical options using prosthetic material (excepting for defect closure with autologous pericardial patch) have very high failure and reintervention rates particularly in younger patients. Advances in translational research and biofabrication hold an unfulfilled promise to save these patients from multiple open replacements of traditional biologic tissue valves and conduits (19,20). In the paediatric population, somatic growth and fate of any implanted prosthetic material exerts a significant effect both on the consequence and frequency matrix of risk (21). Consequence is evaluated by quality-of-life metrics and reoperation-free survival related to biomaterials, whereas frequency could be referred as the complication rate of different types of prosthetic material and/or devices and also the public health burden these reoperations entail.
The objective of the present narrative review is to present current outcomes in paediatric and congenital cardiac surgery in a historical and multidisciplinary context. It represents the paradigm shift in treating congenital heart disease that translated into improved outcomes. Current problems e.g., as in the case of non-viable, non-growing prostheses are identified with the directions of research. We present the following article in accordance with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-47/rc).
Methods
The present narrative overviews landmarks of the pertinent English-language scientific literature (papers and textbooks) and combines them with the latest available (2014–2018) published outcome data from international databases. Programme development and comparative outcome data are presented as a case scenario from a medium-size tertiary-care congenital cardiac centre.
Discussion
Historical context
Paediatric cardiac surgery started with the ligation of a patent arterial duct (PDA) by Robert E Gross in 1939 (22). Creation of an artificial PDA a.k.a. Blalock-Taussig-Thomas shunt (BTS) became a landmark although palliative operation in treating ‘blue babies’, i.e., cyanotic heart disease in the mid-1940s (23). The operation improved symptoms without addressing underlying cardiac pathology, hence the term ‘palliative’. Palliative procedures bring back key aspects of the fetal pattern of circulation, i.e., systemopulmonary shunt acts as an artificial PDA; Blalock-Hanlon atrial septectomy reopens the interatrial communication; and the pulmonary artery banding increases the pulmonary vascular resistance (PVR) to fetal levels. Table 1 demonstrates the comparative characteristics of palliative procedures. Since cardiopulmonary bypass was not available until the 50s, palliation and closed-heart repairs remained the only option but were associated with significant morbidity and mortality (24-26).
Table 1
| Advantages | Disadvantages |
|---|---|
| Gain time for survival | Lose time from anatomical repair |
| Smaller surgery | Cumulative morbidity/mortality of subsequent procedures |
| Overcome critical situation | |
| Modify pathophysiology (induce pulmonary artery growth) | New pathophysiology (pulmonary artery distortion) |
Introduction of medical palliation in the form of prostaglandin E1 (to keep ductal patency) (27,28), Rashkind-balloon atrial septostomy (29) and manipulation of the PVR (30) provided the same ‘fetal’ features, therefore, patients could be stabilized by medical means. These medical modalities reduced the need for palliative procedures and paved the way for primary repair (9). Primary repair involved a strategic and multidisciplinary approach to address the primary pathology rather than complications. The paradigm shift was to perform more complex intracardiac repairs at a younger age (31).
Primary complete repair: key factors of the improvement of outcomes
The key feature of congenital cardiac surgery is that it only exploits its full potential in the conjoint endeavors of a multidisciplinary team. Prerequisites of improved patient outcomes are characterized by significant developments in the allied disciplines highlighted in Table 2.
Table 2
| Discipline | Contribution |
|---|---|
| Cardiac imaging | • Better understanding of cardiac morphology: importance of anatomy increased |
| • Improvements in diagnostics, advanced imaging, emergence of 3D/4D virtual and 3D printed models | |
| • Intraoperative imaging: hybrid OR, TEE | |
| Paediatric cardiology | • Better understanding of the pathophysiological processes |
| • Interventional cardiology: stents, dilatations, hybrid procedures | |
| • Strategic MDT approach | |
| Cardiac surgery | • Problem-centered approach based on better understanding of the pathophysiology and anatomy |
| • Technical aspects: less-invasive surgical approach and technique; standardized surgical steps (e.g., arterial switch operation); better materials, implants, etc. | |
| • CPB improvements: shorter circuit, smaller prime, high hematocrit, moderate hypothermia, selective organ perfusion, improved myocardium protection (cardioplegia) | |
| • Hybrid OR in conjunction with paediatric cardiology and the ‘heart-team’ | |
| Cardiac anesthesia | • Crystallized guidelines and protocols based on better understanding of the pathophysiology: iv. anesthesia, more stable vitals intraoperatively; better non-invasive monitoring; availability of TEE in the OR |
| • Strategic management of iv. and vascular accesses | |
| • iNO, in-OR/early extubation | |
| Intensive care | • Crystallized guidelines and protocols based on better understanding of the pathophysiology (e.g., ventilation protocols, early extubation, early mobilization, etc.) |
| • Proactive management of patient-pathways along expected/observed outcomes | |
| • Widespread availability of perioperative ECMO/ECLS | |
| Institutional | • Organizational aspects: centralization of the caseload and complexity; multidisciplinary approach, teamwork and free communication, case-sharing, etc. Establishment of a centre of excellence based on the triad of treatment-training-research |
| Interdisciplinary | • Risk-stratification, risk-modelling |
| • Creation of international databases, public data reporting |
3D/4D, three-dimensional, four-dimensional; OR, operating room; TEE, transesophageal echocardiography; MDT, multidisciplinary team; CPB, cardiopulmonary bypass; iv, intravenous; iNO, inhaled nitric oxide; ECMO, extracorporeal membrane oxygenation; ECLS, extracorporeal life support.
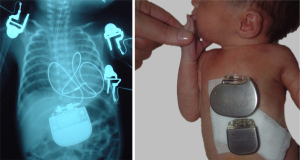
Bedside interventions, e.g., prostaglandin E1 infusion and Rashkind balloon septostomy could stabilize the patient and allow time for procedural planning (32). Improvements in cardiac imaging with the advent of 2D echocardiography in the late 70s grossly increased the importance of cardiac anatomy and paved the way for primary anatomic repair. Nowadays, 3D modelling opens an avenue for preoperative surgical emulation (33). Multidisciplinary approach allows strategic combination of interventional and surgical techniques to work out individual staged procedural plans, e.g., for univentricular case scenarios, complex pulmonary atresia (34). Miniaturization of equipment and prostheses (e.g., pacemakers), consumables contributed to less invasiveness. In comparison to acquired heart disease, the smaller congenital cardiac target population, however, curbs the industry’s financial incentive in development, e.g., in the case of smaller pacemaker generators as shown in Figure 1. The smallest currently available paediatric pacemaker generator came on the market in the early 90s and no significant size reduction is observed after 30 years.
The current state
Suboptimal outcomes became the target of public scrutiny and put paediatric cardiac surgeons rather than surgery in the limelight in the mid-90s (35). The so-called Bristol-affair in the United Kingdom involved a public enquiry that spent millions of pounds and formulated stringent quality recommendations (36). Recommendations for the optimal structure of a congenital heart surgery department emerged that set a minimal annual output of 250 (preferably over 400) operations, a balanced case-mix of index operations (e.g., arterial switch operation), 24/7 accessibility with three (aimed for 4) full-time Consultant surgeons, participation in an international database, etc. as requirements (37). As congenital cardiac surgery deals with the full age range (i.e., from neonates to adults) and associated problems, institutional setup is of relevance. Traditionally, paediatric cardiac surgery was embedded in (I) a paediatric hospital where all the necessary allied disciplines were available; (II) conjoined to large adult cardiothoracic surgical units; or (III)—less frequently—it functioned as a stand-alone, dedicated congenital heart centre (of excellence) providing the full multidisciplinary continuity-of-care. By the nature of the highly specialized discipline, congenital cardiac units are structured in a teaching/university hospital setting. Teaching, training and research is a signifying denominator of large academic centres. Besides adequate caseload and case-mix, staffing, etc., system criteria emerged to ensure the best possible outcomes. These enlist e.g., strong academic leadership, strict departmental routine, regular multidisciplinary meetings and ward-rounds, regular morbidity-mortality audits, reporting to national/international databases. Team performance qualifiers consist of preoperative briefing, time-in and time-out; postoperative multidisciplinary handover, etc. Quality-of-care (system) indicators include availability of extracorporeal membrane oxygenation (ECMO), intraoperative transesophageal echocardiography, etc. Table 3 presents individual quality metrics (key performance indicators) are registered for each patient scenario and are expressed as the expected/observed values.
Table 3
| Quality indicator |
| Major diagnostic mismatch |
| Need for postoperative ECMO/ECLS support |
| Need for postoperative catheter intervention |
| Need for postoperative advanced cardiac imaging |
| Unplanned surgical re-operation (excluding for re-exploration rate for bleeding and delayed sternal closure) |
| Re-exploration for bleeding |
| Delayed sternal closure |
| Phrenic nerve palsy |
| Chylothorax |
| Deep sternal wound infection requiring re-exploration |
| New onset major neurologic deficit including stroke/cerebrovascular accident |
| New onset post-operative renal insufficiency (requiring dialysis/renal replacement therapy) |
| New onset cardiac arrhythmia |
| New onset complete heart block (after surgery necessitating permanent pacemaker insertion) |
| Operative mortality reported by complexity/risk stratification class |
ECMO, extracorporeal membrane oxygenation; ECLS, extracorporeal life support.
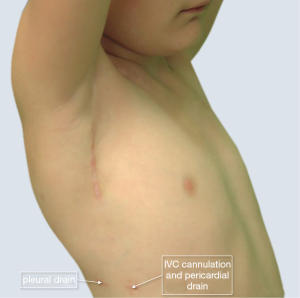
Minimally invasive techniques derive from adult cardiothoracic surgery (38) and they find their application in congenital cardiac surgery per ‘virtu de necessitate’ (virtue of out necessity) (39). These methods have had so far limited place due to complexity, need to access multiple segments in a constrained operative field (40). Minimally invasive surgery, complemented with augmented visualization (e.g., video-assisted surgery) however offers several advantages in reducing morbidity and more appealing cosmesis without jeopardizing quality of the repair and safety. A wide variety of lesions ranging from resection of subaortic stenosis, atrial and/or ventricular septal defect (ASD/VSD)-closure to mitral surgery are now routinely performed with minimally/less invasive techniques, e.g., from right subaxillary, muscle-sparing thoracotomy (41,42) (Figure 2).
Hybrid cardiac surgery/catheter strategies emerge as new modality where the surgeon allowing access and performing parts of the procedure and the invasive cardiologist employing catheter interventional methods complements each other in tandem fashion (43). Hybrid procedures are gaining an evolving role as treatment strategy for selected congenital cardiac patients. The benefits of this approach are directly translated in improved patient outcomes, by limiting the sternotomies, reducing morbidity and LOS.
Risk stratification and risk modelling. Congenital cardiac surgery applies a wide spectrum of procedures for an even wider spectrum of anomalies and their combinations. Furthermore, there is a broad variation of age, weight, preoperative conditions affecting the outcome. Stratification of the operative risk is the first step towards risk modelling, i.e., to estimate the risk for the individual patient. Chronologically, Aristotle Basic Complexity (ABC) scoring was introduced first in 2002 that weighted expert opinion (doxa) of the potential mortality, morbidity and technical difficulty (44,45). The ABC-scoring system classifies 167 distinct procedures into 4 Levels and assigns a continuous value from 1.5 (e.g., pericardiocentesis) to 15 (e.g., biventricular repair of hypoplastic left heart). The Comprehensive Aristotle Score introduces patient-adjusted complexity by including age, co-morbidity, clinical status etc. and steps forward to risk modelling (46). The Risk Adjustment in Congenital Heart Surgery (RACHS-1) system sets six different risk categories based on expert judgment and on empirical data (discharge mortality) (47-49). The latest in the line, Society of Thoracic Surgeons (STS)/European Congenital Heart Surgeons Association (ECHSA) Congenital Heart Surgery Mortality Categories (STAT)—introduced in 2010—utilizes statistical estimation, i.e., an empirical methodology of risk stratification of objective data from the respective STS/ECHSA databases (50,51). STAT data originally derived from 2002–2007 database entries, and so, this most widely used empirically-based tool has been updated (52). The new 2020 Mortality Scores and Categories allow for accurate, up-to-date assessment of case-mix, which is an essential element of outcome reporting, quality assessment, and quality improvement initiatives. International databases (53,54) are available and subscription to one is now a key performance requirement for any congenital cardiac surgery centre. Risk stratification has clearly moved away from the opinion-based to evidence-based standpoint, and inclusion of morbidity as well as patient-specific parameters (e.g., age, weight, perioperative mechanical circulatory support, renal failure, associated syndromes, etc.) promote proper risk modelling.
Full transparency on congenital heart surgery outcomes is promoted from the public, healthcare regulator, financier and patient-groups and it is fully agreed by the surgical community (55). Public reporting, however, leads to risk-averting behavior, limits access to healthcare; self-reporting physicians tend to upgrade the risk category (56,57). Publication of institutional rather than personal outcomes could overcome the bias and could also put an emphasis on interdisciplinary teamwork and cooperation.
In congenital cardiac surgery adverse surgical outcome is closely associated with low hospital and surgeon annual caseload (37,58,59). There is a wide spectrum of distinct anatomies and almost unlimited combination operative scenarios with increasing complexity. In a setting, where complex operations are in low volume and/or are rarely performed, it is more difficult to reach and maintain the critical mass of experience for the entire multidisciplinary team, not only for surgeons. Financial directives dictated by low volumes deprive adequate staffing and the availability uninterrupted multidisciplinary services (60). A low caseload and case-mix prevents adequate training for younger surgeons, in a world, where adverse outcomes are neither expected nor tolerated (35,36). The steep learning curve and consistently maintained results both at individual and team level could be ameliorated by proper mentoring, readily available at larger centres (61,62). All these aspects prompt towards centralization into large paediatric cardiac centres with adequate surgical output and case-mix, and multidisciplinary staffing, comprehensive allied and supporting services. Centralization remains a debated topic, although it has been successfully performed in Sweden (63), and initiated in the United Kingdom (64), and is theoretically discussed in literature in the United States (65).
Finally, congenital cardiac surgery developed in socioeconomically advantaged countries and there are gross geographical differences, leaving—according some estimates—90% of CHD patients without access to cardiac care (66). Charity programmes have been organized to help underprivileged areas, however, these typically short missions neither addressed the long-term view of the continuum-of-care, nor the training of the local professionals; so, the trips were often labelled as “surgical safaris” (67). Education of trainees at high-volume, high-quality centres and provision of continuing coaching is a more successful avenue (68). Programme evolution and maturation can be closely monitored by ongoing mutual visits of the involved teams (69). Thirdly, (senior) professional(s) move locally to establish and develop a new treatment centre. In this scenario, clinical commitments and educational aspects could be fulfilled hand-in-hand (60,70). In a following section, we present an example of the third scenario.
Outcome analysis of specific types of procedures
Between 2015 and 2018, STS Congenital Heart Surgery Database enlists 64,942 total operations (without chest closures and explorations) with an overall procedural mortality 2.5% (2.3–2.7, 95% CI) (71). The most common types of procedures: repair of VSD, ASD and complete atrioventricular septal defect (cAVSD) carry low mortality (respectively, 0.5%, 0.2%, and 2.0%). Modified Norwoood-1 operation is the fourth most frequently performed procedure with the highest hospital mortality of 13.2% in the series. Classical palliative procedures—pulmonary artery banding, systemopulmonary shunt—closely follow it with high mortality rates of 7.1% and 7.4% (8.1% for central shunts). One could conclude that stage 1 procedure for HLHS does not resolve but creates unreliable haemodynamics at a very delicate physiological balance; and palliative procedures—as mentioned—do not address morphologic repair. In contrast, repair of tetralogy of Fallot is performed with a mortality rate of 0.5% (without ventriculotomy), 0.6% (with ventriculotomy) and 1.7% (with transannular patching). Whenever sustainable haemodynamics can be achieved, survival of even the complex operations is well above 98%. Overall mortality of 6.6% with a repair of total anomalous pulmonary venous drainage stands out from the series that points to ongoing associated pulmonary vascular pathology. The role of comorbidities is further exemplified by an overall mortality rate of 4.2% associated with surgical PDA ligation. We conclude that suboptimal outcome cannot be labelled as simply surgical but rather multisystem failure in both pathophysiological and clinical aspects. Several specific conditions merit further observations:
The arterial switch operation for d-transposition of the great arteries (TGA) is the ultimate success story in congenital cardiac surgery (72). For an entity with a universally fatal natural history anatomic correction was tried first, without success, by Mustard and Senning in the mid-50s. Later on, the same authors independently developed atrial switch operation that provided physiological repair (73,74). Despite the atrial switch operation carried lower procedural risks, long-term failure of the systemic right ventricle, atrial arrhythmia and deteriorating functional status of the survivors prompted for anatomic correction, first successfully performed by Jatene et al. in 1976 (31). Initial operative mortality of the arterial switch operation was much higher (18%) than what was achieved with the atrial switch (4%). As the steps of the arterial switch operation (75) and especially the coronary transfer became standardized, results dramatically improved (76,77). Nowadays, most centres perform this operation with near-zero mortality (78). There are several key factors in the success: TGA presents with relatively uniform anatomy and physiology that makes it suitable to stereotype the treatment strategy; surgical steps and caveats are also highly standardized (79). The history of treatment for TGA and especially of the arterial switch operation provides a context for considering the ethics of surgical innovation in the current era (56,57).
Intracardiac repair combined with the repair of extracardiac structures (e.g., aortic arch, pulmonary artery reconstruction, tracheobronchial surgery, etc.) denotes that complete repair needs to address several cardiac segments and operative areas at the same operation. Midline one-stage complete unifocalization and repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals (80), intracardiac repair with aortic arch reconstruction (e.g., Taussig-Bing anomaly with transverse aortic arch hypoplasia) (81), etc. enlists here. These complex procedures carry an increased risk of morbidity and mortality (47) that can be reduced by individual decision-making, strategic cooperation between e.g., interventional cardiologist and the surgeon, and by the application of selective cerebral and myocardial perfusion techniques (82).
Survival of modified Norwood 1 operation for hypoplastic left heart complexes has significantly improved, and the most experienced centers now report hospital survivals above 90% (83). Current trends favor RV-to-PA conduit over modified BTS (84). Despite several modification in perfusion strategy (85) and in the postoperative care hospital survival somewhat plateaued since the 2000s (68). Interstage mortality exceeds hospital mortality and remains at the highest of 10–25% (86) prompting strict surveillance programmes (87). Hybrid stage 1 with selective pulmonary artery banding and with/out PDA stenting have been proposed to altogether avoid high-risk open-heart surgery on cardiopulmonary bypass (i.e., modified Norwood-1 procedure) in a neonate (88). Hybrid approach, however, did not change overall survival at the second stage and failed to improve worse neurodevelopmental outcomes associated with hypoplastic left heart (89). Furthermore, pulmonary branch distortion frequently accompanying selective pulmonary bands may even jeopardize the successful transition to bidirectional superior cavopulmonary anastomosis at comprehensive stage-2 operations (90).
Univentricular staging: STS Congenital Heart Surgery Database quotes a hospital mortality rate of 1.7% for superior cavopulmonary anastomosis (BDG; 1.8% with concomitant pulmonary artery plasty) and 1.3% for total cavopulmonary connection (TCPC; when fenestrated, as in 57.3% of cases) and 0.5% (without fenestration) (71). Traditional knowledge puts TCPC completion at a higher mortality rate than the one with BDG. Lower mortality associated with TCPC completion featured in STS Congenital Database argue for possibly improved staging protocols and appropriate patient selection for the final procedure.
Reoperations: As premised, about thirty percent of the CHD patients need a staged operating plan for physiological and/or anatomical reasons. Currently available biomaterials lack the potential of growth, and conduits, valves derange over time surrendering patients to reoperations. At present, a neonate/infant with conduit or valve implantation undergoes 2–3 reoperations before reaching adulthood for structural failure or for simply growing out the implant (91). Cardiac surgery for adult congenital heart patients currently represents 20% of the entire congenital cardiac surgery activity and most these procedures are reoperations (64). This is a huge public health burden (4). Availability of viable, growing biomaterials could cancel reoperations from the start, and that could entail with significant public health benefit and improved quality-of-life (19).
Case presentation: how to establish a new tertiary-care, comprehensive programme in congenital cardiac surgery
In the following section, aspects of development of a new comprehensive programme for CHD are highlighted in the unique setting of the United Arab Emirates (UAE). Knowledge could be expanded to other countries in the region, although high-volume/high quality services and a long tradition in paediatric cardiac surgery exist e.g., in the Kingdom of Saudi Arabia, Qatar and Kuwait. Historically, Emirati CHD patients were sent abroad for surgery; and no dedicated comprehensive treatment facility existed for resident non-nationals in the UAE. The country has a peculiar social context where indigenous Emiratis (15-18% of the country’s total population) and resident immigrants form separated population groups with little reproductive mixing (92). Consanguinity is traditionally and culturally prevalent (93). Birth rate gradually decreases but remains higher than the European average (94). Religious creeds do not allow termination of pregnancy (95). All these aspects contribute to an increased prevalence of CHD, birth anomalies and especially enhance their complexity (96). Infant mortality rate radically decreased and now reaches to the range of the high-income countries (1950: 178/1,000 to 2015: 10.59/1,000 live births), signifying a rapidly advancing health care infrastructure (97). Public need prompted the foundation of a comprehensive service for CHD at Sheikh Khalifa Medical City, Abu Dhabi (SKMC)—a 586-bed, acute-care, governmental, flagship facility of UAE healthcare—in 2007. The tertiary-care congenital cardiac programme was embedded in the setting of a cardiac institute, alongside to adult cardiology/cardiac surgery and a paediatric department. The author has been a programme initiator and leader of the surgical service for fifteen years that provides a personal perspective of the strategies suitable for generalization and adaptation elsewhere by framing outcomes in terms of global trends relating to congenital cardiac surgery.
Besides the international recommendations for congenital cardiac centres (37,98), various strategic, tactical and operational models were employed to develop the specifics of a comprehensive service for CHD. Relations with the institutional environment and the local professional and social network (e.g., sociocultural aspects) were closely observed. In the strategic planning/designing phase of the programme, patient pathways, scenario-planning, continuum-of-care plan, etc. were drawn up (99). The operational phase was modelled along strong departmental leadership, rigid professional routines, multidisciplinary teamwork and communication (100). For quality control, we applied international outcome benchmarks, continuous reporting of key performance indicators (as in Table 3), and the strategic model of strengths-weaknesses-opportunities-threats (SWOT) analysis (101). Root cause analysis of expected/observed outcome differences was operational to establish a plan-do-check-act cycle (102). Importantly, pertinent UAE Health Law defined congenital heart disease as a ‘life or limb emergency’ that provided a strong mandate to treat all residents, and thus, it ensured the financial sustainability of the service (103).
The comprehensive service encompassed 24/7 availability of the continuum-of-care of all diagnostic, catheter-based interventional, hybrid and surgical arms along with ECMO/ECLS. The programme attracted nationwide referrals and developed into a national, tertiary-care provider for CHD represented on Figure 3.
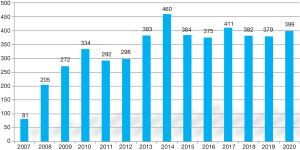
Observation of the annual volumes demonstrates linear growth in the formative years of the programme followed by a plateaud phase owing to a number of intrinsic and extrinsic factors. Among the intrinsic factors we mention the lack of organization of all allied disciplines into an unified paediatric cardiac department, but rather the multidisciplinary team—still belonging to their own organizational structures—functioning by voluntary cooperation. A consecutive lack of a conjoint development plan for treatment facilities and team hampered the service’s evolution into a regional centre of excellence. On the other hand, a very cohesive professional team (with very little fluctuation of key medical professionals) and the spirit of ownership corroborated programme development. As countrywide healthcare infrastructure developed and general insurance coverage became mandatory, the government gradually stiffened eligibility criteria for mandated care that had a significant extrinsic effect on patient care, especially in financial coverage for staged reoperations. It also tilted the case-mix towards the neonatal and infant patient population. Distribution of age-groups, complexity and acuity of the surgical procedures is presented on Table 4.
Table 4
| Age groups | Neonates | 1 month – 1 year | Beyond 1 year | Statistical significancea |
|---|---|---|---|---|
| N=4,655; primary procedures | 1,499 (32.2%) | 1,810 (38.9%) | 1,346 (28.9%) | Emergency neonates vs. electives beyond 1 year |
| Elective | 158 | 1,091 | 1,102 | OR: 366.08 |
| Urgent | 1,021 | 596 | 190 | P value: 0.001 |
| Emergency | 320 | 123 | 54 | |
| Complexity (mean, SD) | 8.78 SD2.84 | 7.45 SD2.14 | 7.05 SD2.32 | t-value: 7.21381; |
| P value: 0.00001 | ||||
| Survival (%) | 96.92 | 97.93 | 98.24 | OR: 2.46; P value: 0.116; NS |
“Elective” is defined as the surgical procedure is to be performed in the preferred time-frame; “urgent”: within the same hospitalization; “emergency”: within 24–48 hours. Complexity is displayed on a continuous Aristotle Basic Complexity range of 1.5–15. a, a two-tailed, paired Student’s t-test was used for the comparison of numerical variables. P value <0.05 was considered statistically significant. Categorical data were analysed by khi-square test and odds-ratio is provided (OR). OR, odds ratio; SD, standard deviation; NS, non significant.
Data reveal two distinct cohorts: (I) neonates and (II) patients above 1 year of age with a significantly higher complexity and acuity among the neonates. Survival, however, has not been significantly different between the groups. Higher representation of neonates and patients less than 1 year of age (71.1%) may explain the overall higher complexity when comparing historical local data with international dataset (104) (Table 5).
Table 5
| SKMC data | STS-CHSD database | ||||
|---|---|---|---|---|---|
| Distribution | Mortality | Distribution | Mortality | ||
| RACHS-1 Level 1 | 12.99 | 0.24 | 13.37 | 0.63 | |
| RACHS-1 Level 2 | 33.86 | 1.5 | 38.74 | 1.38 | |
| RACHS-1 Level 3 | 32.28 | 4.27 | 32.31 | 4.09 | |
| RACHS-1 Level 4 | 14.17 | 5.56 | 10.70 | 8.66 | |
| RACHS-1 Level 5 & 6 | 8.66 | 9.09 | 4.89 | 20.15 | |
| ABC Class 1 | 13.78 | 1.43 | 16.17 | 1.62 | |
| ABC Class 2 | 34.65 | 1.70 | 43.28 | 2.56 | |
| ABC Class 3 | 29.92 | 2.63 | 27.06 | 4.09 | |
| ABC Class 4 | 21.65 | 5.45 | 13.49 | 9.95 | |
SKMC, Sheikh Khalifa Medical City, Abu Dhabi; STS-CHSD, Society of Thoracic Surgeons Congenital Heart Surgery Database; RACHS-1, Risk Adjustment in Congenital Heart Surgery score system; ABC, Aristotle Basic Complexity score.
Contemporary SKMC crude mortality (2015–2018: 2.68%) is comparable to the large dataset from international benchmarks [NICOR (UK) 2014–2017: 2.82%] (105) (Table 6).
Table 6
| Surgical procedure/mortality | SKMC (2016–2018) | NICOR (UK, 2014–2017) | STS-CHSD (US, 2012–2018) |
|---|---|---|---|
| VSD Closure | 0% | 0.1% | 0.6% |
| TOF | 0% | 0.5% | 0.9% |
| cAVSD | 0% | 1.5% | 2.9% |
| Bidirectional Glenn | 0% | 1.9% | 2.4% |
| Fontan Procedure | 0% | 0.7% | 1.2% |
| Aortopulmonary shunts | 4.5% | 9.1% | 7.8% |
| Arterial switch VSD closure | 0% | 0.7% | 5.4% |
| Arterial switch + arch repair | 0% | 10% | – |
| Norwood stage 1 procedure | 13.3% | 10.4% | 15.5% |
SKMC, Sheikh Khalifa Medical City, Abu Dhabi; NICOR, National Institute for Cardiovascular Outcomes Research; STS-CHSD, Society of Thoracic Surgeons Congenital Heart Surgery Database; VSD, ventricular septal defect; TOF, tetralogy of Fallot; cAVD, complete atrioventricular septal defect.
Due to the peculiar sociodemographic characteristics of the UAE, the adult congenital heart disease (ACHD) patient population is still small (less than 2.5% in our dataset) and its gradual increase is expected once the patients operated on by the SKMC programme reach adolescent age (107).
Effect of the COVID-19 pandemic on the treatment of CHD. SKMC’s paediatric cardiac programme remained fully functional and surgical volume slightly increased during the COVID-19 pandemic in 2020. The pandemic accelerated transformation of the healthcare structure in general, and in CHD care in particular. Several key shifts changes are observed to meet public health demand in treating CHD:
- As personal encounters diminished, importance of telemedicine increased. Our team developed robust home-monitoring programme using telemedicine and digital consultation.
- The decrease of the inpatients resulted in opening of in-house bed capacity. Most of the allied team was reassigned to look after COVID-19 patients, however the core cardiac team was retained for their special expertise in treating CHD.
- Communication with referring centres and physicians and hospitals intensified that progressed to a more integrated healthcare system, where resources, teams and facilities could be shared.
- Referral routes crystallized in association to a rapid halt in international medical tourism.
- As a consequence of (IV) and (V), acute referrals increased
- In general: as elective surgery decreases, fixed-cost increases that is better tolerated by larger, academic and financially stable institutions.
The pandemic seems to accelerate transformation; the worldwide trend is already in progress towards larger, comprehensive programmes that can exploit enhanced communication tools (e.g., virtual meetings, multi-institutional cooperation, telemedicine, etc.) (108).
We summarize with a SWOT analysis that the service has its strenghts as an established provider, recognized by both the professional and public community. It has a cohesive multidisciplinary team that offers multimodality treatment (e.g., hybrid procedures) for the entire spectrum of age and complexity. The weaknesses are related to the high case-mix index that is labour- and cost-intensive, staff-, equipment sensitive and, therefore, is expensive. Respective divisions belong to different departments that weakens executive action. Thus, the service remains reliant on the voluntary cooperation of the allied disciplines and support services. Availability of comprehensive treatment modalities: neonatal open-heart surgery, hybrid programme, ACHD/GUCH-programme, ECMO, etc. create opportunities to become a centre of excellence and to grow from a small-medium size provider into a large-size, regional one. The service-model is suitable for receiving overseas trainees and it presents franchise opportunity (i.e., establishment of affiliated centres). Lack of a long-term strategic development plan and a constantly changing eligibility (patient-access) environment are considered as threats.
In concluding the observations on the SKMC programme development, we propose the following conclusions transferable to other developing programmes and countries: (I) a successful program should carry a strong spirit of ownership based on transparent communication of the multidisciplinary team; (II) strict professional guidelines and rigid teamwork routines are mandatory; (III) the program should meet a public health demand that provides adequate caseload and case-mix; (IV) a robust quality control system needs to be implemented with continuous recording and reporting of the expected/observed outcomes; (V) the programme should be accessible for all CHD patients from the referral area along the entire continuum-of-care, in 24/7, in which, (VI) financial sustainability is fundamental that warrants a solid leadership/governmental mandate.
Future trends
Paediatric and congenital cardiac surgery travelled a long journey over a short period of time. Blossoming branch of this subspecialty sprouted off from the trunk of general surgery via cardiothoracic surgery some sixty years ago. Subspecialisation has no doubt contributed to the current excellent results (64) (Figure 4).
The specialty was recognized as a separate discipline in 2000s (in 2013 in the United Kingdom) (64). First congenital surgeons spent considerable time in the surgical laboratory establishing the prerequisites of cardiopulmonary bypass and the surgical technique as well as in the pathology museum to study pathomorphology. Current surgeons interact with a multidisciplinary team by becoming proficient at hybrid/catheter-based interventions. The next generation of surgeons may well be versed with video-gaming and virtual reality that can be exploited in minimally invasive: endoscopic, robotic and hybrid procedures. Or the new generation of surgeons may come from the scientific labs and capitalize on tissue-regeneration techniques.
Procedural risk related to congenital cardiac surgery is still high. Furthermore, a significant proportion of patients cannot be treated by a single procedure. At present, there is no possibility to regenerate or complement missing cardiovascular segments with a living and growing construct. This cluster of problems is registered at a public health level as an increasing number of patients live with ‘repaired’ congenital heart disease. On one hand, it is expected the trend will continue ‘preventing’ complex congenital heart disease by termination of affected pregnancies (109). The need for ‘open’ congenital cardiac surgical operations, particularly complex neonatal surgery will continue in the foreseeable future. On the other, it is hoped that emerging modalities, e.g., augmented surgical visualization, virtual reality and artificial intelligence, along with the application of microrobots could reduce the significant aggression and morbidity associated with open-heart surgery. Xenotransplantation attempting to outwit evolution holds the promise of an unlimited pool of suitable organs and tissues, however, experimental results of cell xenotransplantation, e.g., islet or neuronal cells, are currently significantly better than those of organ xenotransplantation (110). Additionally, better biomaterials from nanotechnology, 3D-bioprinting and bioengineering of cells and tissue architectures could revolutionize regenerative medicine and congenital cardiac surgery in particular (111). Evolving technologies can combine bioprinting and bioassembly by perfecting composite biomaterials and autologous stem cells, may also include natural signaling cues and replay developmental processes. Thus, it is expected that congenital cardiac surgery—as we know it today with repeated sternotomies, extensive procedures on full cardiopulmonary bypass, deep hypothermia, selective organ perfusion and/or total circulatory arrest, etc., and thus, carrying substantial biological aggression and hazard—one day will become obsolete.
Summary
Congenital cardiac surgery remains one of the most demanding and technically complex areas in cardiothoracic surgery. It is also one of the most rewarding as successful surgery is not just life-saving but also offers many young patients the chance of a long and good quality life (64). Procedural risk related to congenital cardiac surgery is still high. Advanced knowledge of the underlying pathophysiology, establishment of individualized care-plans, improved technology and techniques, multidisciplinary effort has contributed to significantly improved survival and quality-of-life expectations. Minimal invasive techniques, hybrid-procedures, advent of biofabrication of viable and growing implants will hopefully reduce biological aggression and morbidity while ensuring sustainable excellent outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Swee Chye Quek and Yiu-Fai Cheung) for the series “Advances in Pediatric Cardiology” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-47/rc
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-47/coif). The series “Advances in Pediatric Cardiology” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Congenital heart disease statistics 2006. British Heart Foundation, Available online: www.heartstats.org, retrieved on 20 March 2008.
- Oster ME, Kim CH, Kusano AS, et al. A population-based study of the association of prenatal diagnosis with survival rate for infants with congenital heart defects. Am J Cardiol 2014;113:1036-40. [Crossref] [PubMed]
- Hoffman JIe. The global burden of congenital heart disease. Cardiovasc J Afr 2013;24:141-5. [Crossref] [PubMed]
- GBD 2017 Congenital Heart Disease Collaborators. Global, regional, and national burden of congenital heart disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc Health 2020;4:185-200. [PubMed]
- Daebritz SH. Update in adult congenital cardiac surgery. Pediatr Cardiol 2007;28:96-104. [Crossref] [PubMed]
- Webb GD. Challenges in the care of adult patients with congenital heart defects. Heart 2003;89:465-9. [Crossref] [PubMed]
- Wren C, O'Sullivan JJ. Survival with congenital heart disease and need for follow up in adult life. Heart 2001;85:438-43. [Crossref] [PubMed]
- Tynan MJ, Becker AE, Macartney FJ, et al. Nomenclature and classification of congenital heart disease. Br Heart J 1979;41:544-53. [Crossref] [PubMed]
- Higashi H, Barendregt JJ, Vos T. The burden of congenital anomalies amenable to surgeries in low-income and middle-income countries: a modelled analysis. Lancet 2013;381:S62. [Crossref]
- Castaneda AR, Mayer JE Jr, Jonas RA, et al. The neonate with critical congenital heart disease: repair--a surgical challenge. J Thorac Cardiovasc Surg 1989;98:869-75. [Crossref] [PubMed]
- Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax 1971;26:240-8. [Crossref] [PubMed]
- Norwood WI, Kirklin JK, Sanders SP. Hypoplastic left heart syndrome: experience with palliative surgery. Am J Cardiol 1980;45:87-91. [Crossref] [PubMed]
- Zimmermann WH, Cesnjevar R. Cardiac tissue engineering: implications for pediatric heart surgery. Pediatr Cardiol 2009;30:716-23. [Crossref] [PubMed]
- Delius RE, Rademecker MA, de Leval MR, et al. Is a high-risk biventricular repair always preferable to conversion to a single ventricle repair? J Thorac Cardiovasc Surg 1996;112:1561-8; discussion 1568-9. [Crossref] [PubMed]
- Ohuchi H. Adult patients with Fontan circulation: What we know and how to manage adults with Fontan circulation? J Cardiol 2016;68:181-9. [Crossref] [PubMed]
- d'Udekem Y, Iyengar AJ, Cochrane AD, et al. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation 2007;116:I157-64. [Crossref] [PubMed]
- The Society of Thoracic Surgeons Congenital Heart Surgery Data Summary, All Patients STS Period Ending 12/31/2018. Available online: https://www.sts.org/sites/default/files/Congenital-STSExecSummary_AllPatients.pdf, retrieved on 15 December 2020.
- Coulson JD, Seddon MR, Readdy WF. Advancing Safety in Pediatric Cardiology—Approaches Developed in Aviation. Congen Cardiol Today 2008;6:1-10.
- Galantowicz M. Encouraging six-month results with Gore novel biosynthetic tissue valve. Available online: https://www.prnewswire.com/news-releases/w-l-gore--associates-announces-encouraging-six-month-results-with-its-novel-biosynthetic-tissue-valve-301180050.html, retrieved 15 December 2020.
- Kiraly L, Vijayavenkataraman S. Biofabrication in Congenital Cardiac Surgery: A Plea from the Operating Theatre, Promise from Science. Micromachines 2021;12:332. [Crossref] [PubMed]
- Sutton I. Process Risk and Reliability Management, 2nd Edition. Elsevier, Amsterdam, 2015:17.
- Gross RE, Hubbard JP. Landmark article Feb 25, 1939: Surgical ligation of a patent ductus arteriosus. Report of first successful case. By Robert E. Gross and John P. Hubbard. JAMA 1984;251:1201-2. [Crossref] [PubMed]
- Blalock A, Taussig HB. Landmark article May 19, 1945: The surgical treatment of malformations of the heart in which there is pulmonary stenosis or pulmonary atresia. By Alfred Blalock and Helen B. Taussig. JAMA 1984;251:2123-38. [Crossref] [PubMed]
- Holman WL, Buhrman WC, Oldham HN, et al. The Blalock-Taussig shunt: an analysis of trends and techniques in the fourth decade. J Card Surg 1989;4:113-24. [Crossref] [PubMed]
- Cornell WP, Maxwell RE, Haller JA, et al. Results of the Blalock-Hanlon operation in 90 patients with transposition of the great vessels. J Thorac Cardiovasc Surg 1966;52:525-32. [Crossref] [PubMed]
- LeBlanc JG, Ashmore PG, Pineda E, et al. Pulmonary artery banding: results and current indications in pediatric cardiac surgery. Ann Thorac Surg 1987;44:628-32. [Crossref] [PubMed]
- Heymann MA, Berman W, Rudolph AM, et al. Dilatation of the ductus arteriosus by prostaglandin E1 in aortic arch abnormalities. Circulation 1979;59:169-73. [Crossref] [PubMed]
- Neutze JM, Starling MB, Elliott RB, et al. Palliation of cyanotic congenital heart disease in infancy with E-type prostaglandins. Circulation 1977;55:238-41. [Crossref] [PubMed]
- Rashkind WJ, Miller WW. Creation of an atrial septal defect without thoracotomy. A palliative approach to complete transposition of the great arteries. JAMA 1966;196:991-2. [Crossref] [PubMed]
- Mitzner W. Resistance of the pulmonary circulation. Clin Chest Med 1983;4:127-37. [Crossref] [PubMed]
- Jatene AD, Fontes VF, Paulista PP, et al. Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg 1976;72:364-70. [Crossref] [PubMed]
- Akkinapally S, Hundalani SG, Kulkarni M, et al. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst Rev 2018;2:CD011417. [Crossref] [PubMed]
- Yoo SJ, Thabit O, Kim EK, et al. 3D printing in medicine of congenital heart diseases. 3D Print Med. 2015;2:3.
- Barron DJ, Botha P. Approaches to Pulmonary Atresia With Major Aortopulmonary Collateral Arteries. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2018;21:64-74. [Crossref] [PubMed]
- Dunn PM. The Bristol affair. Surgeons were treated unjustly. BMJ 1998;317:1659-60. [Crossref] [PubMed]
- Monro JL. Lessons to be learnt from the Bristol affair. Ann Thorac Surg 2000;69:674-5. [Crossref] [PubMed]
- Daenen W, Lacour-Gayet F, Aberg T, et al. Optimal structure of a congenital heart surgery department in Europe. Eur J Cardiothorac Surg 2003;24:343-51. [Crossref] [PubMed]
- Kofidis T. Minimally Invasive Cardiac Surgery. A Practical Guide. CRC Press: Boca Raton FL, USA, 2021.
- del Nido PJ. Minimal incision congenital cardiac surgery. Semin Thorac Cardiovasc Surg 2007;19:319-24. [Crossref] [PubMed]
- Villa E, Vanden Eynden F, Le Bret E, et al. Paediatric video-assisted thoracoscopic clipping of patent ductus arteriosus: experience in more than 700 cases. Eur J Cardiothorac Surg 2004;25:387-93. [Crossref] [PubMed]
- Yang X, Wang D, Wu Q. Repair of atrial septal defect through a minimal right vertical infra-axillary thoracotomy in a beating heart. Ann Thorac Surg 2001;71:2053-4. [Crossref] [PubMed]
- Wang Q, Li Q, Zhang J, et al. Ventricular septal defects closure using a minimal right vertical infraaxillary thoracotomy: seven-year experience in 274 patients. Ann Thorac Surg 2010;89:552-5. [Crossref] [PubMed]
- Bacha E, Kalfa D. Minimally invasive paediatric cardiac surgery. Nat Rev Cardiol 2014;11:24-34. [Crossref] [PubMed]
- Lacour-Gayet F, Clarke D, Jacobs J, et al. The Aristotle score: a complexity-adjusted method to evaluate surgical results. Eur J Cardiothorac Surg 2004;25:911-24. [Crossref] [PubMed]
- Jacobs JP, Mayer JE Jr, Mavroudis C, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2017 Update on Outcomes and Quality. Ann Thorac Surg 2017;103:699-709. [Crossref] [PubMed]
- O’Brien SM, Jacobs JP, Clarke DR, et al. Accuracy of the Aristotle Basic Complexity Score for Classifying the Mortality and Morbidity Potential of Congenital Heart Surgery Operations. Ann Thorac Surg 2007;84:2027-37. [Crossref] [PubMed]
- Jacobs JP, Jacobs ML, Lacour-Gayet FG, et al. Stratification of Complexity Improves the Utility and Accuracy of Outcomes Analysis in a Multi-Institutional Congenital Heart Surgery Database: Application of the Risk Adjustment in Congenital Heart Surgery (RACHS-1) and Aristotle Systems in the Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database. Pediatr Cardiol 2009;30:1117. [Crossref] [PubMed]
- McSharry B, Straney L, Alexander J, et al. RACHS - ANZ: A Modified Risk Adjustment in Congenital Heart Surgery Model for Outcome Surveillance in Australia and New Zealand. J Am Heart Assoc 2019;8:e011390. [Crossref] [PubMed]
- Al-Radi OO, Harrell FE, Caldarone CA, et al. Case complexity scores in congenital heart surgery: A comparative study of the Aristotle Basic Complexity score and the Risk Adjustment in Congenital Heart Surgery (RACHS-1) system. J Thorac Cardiovasc Surg 2007;133:865-75. [Crossref] [PubMed]
- O'Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg 2009;138:1139-53. [Crossref] [PubMed]
- Jacobs JP, Jacobs ML, Maruszewski B, et al. Initial application in the EACTS and STS Congenital Heart Surgery Databases of an empirically derived methodology of complexity adjustment to evaluate surgical case mix and results. Eur J Cardiothorac Surg 2012;42:775-9; discussion 779-80. [Crossref] [PubMed]
- Jacobs ML, Jacobs JP, Thibault D, et al. Updating an Empirically Based Tool for Analyzing Congenital Heart Surgery Mortality. World J Pediatr Congenit Heart Surg 2021;12:246-81. [Crossref] [PubMed]
-
Congenital Database ECHSA - STS Congenital Heart Surgery Database. Available online: https://www.sts.org/registries-research-center/sts-national-database/congenital-heart-surgery-database, retrieved 20 April 2021.
- Welke KF. Public Reporting of Congenital Heart Surgery Outcomes: Counting Numbers, Caring for Patients. Semin Thorac Cardiovasc Surg 2018;30:50-3. [Crossref] [PubMed]
- Spray TL, Gaynor JW. A Word of Caution in Public Reporting. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2017;20:49-55. [Crossref] [PubMed]
- Tweddell JS. A nondystopian alternative history? J Thorac Cardiovasc Surg 2018;156:773-4. [Crossref] [PubMed]
- Jenkins KJ, Newburger JW, Lock JE, et al. In-hospital mortality for surgical repair of congenital heart defects: preliminary observations of variation by hospital caseload. Pediatrics 1995;95:323-30. [Crossref] [PubMed]
- Hraska V, Podnar T, Kunovsky P, et al. Is a learning curve for arterial switch operation in small countries still acceptable? Model for cooperation in Europe. Eur J Cardiothorac Surg 2003;24:352-7. [Crossref] [PubMed]
- Jenkins KJ, Castañeda AR, Cherian KM, et al. Reducing mortality and infections after congenital heart surgery in the developing world. Pediatrics 2014;134:e1422-30. [Crossref] [PubMed]
- Cohen MS, Jacobs JP, Quintessenza JA, et al. Mentorship, learning curves, and balance. Cardiol Young 2007;17:164-74. [Crossref] [PubMed]
- Mussa S, Drury NE, Stickley J, et al. Mentoring new surgeons: can we avoid the learning curve?†. Eur J Cardiothorac Surg 2017;51:291-9. [PubMed]
- Lundström N, Berggren H, Björkhem G, et al. Centralization of Pediatric Heart Surgery in Sweden. Pediatr Cardiol 2000;21:353-7. [Crossref] [PubMed]
- UK Cardiothoracic Surgery. SAC and SCTS Workforce report 2019. Available online: https://scts.org/wp-content/uploads/2019/01/SCTS-workforce-report-2019.pdf, retrieved 20 April 2021.
- Welke KF, Pasquali SK, Lin P, et al. Regionalization of Congenital Heart Surgery in the United States. Semin Thorac Cardiovasc Surg 2020;32:128-37. [Crossref] [PubMed]
- Murala JSK, Karl TR, Pezzella AT. Pediatric Cardiac Surgery in Low-and Middle-Income Countries: Present Status and Need for a Paradigm Shift. Front Pediatr 2019;7:214. [Crossref] [PubMed]
- Corno AF. Paediatric and congenital cardiac surgery in emerging economies: surgical 'safari' versus educational programmes. Interact Cardiovasc Thorac Surg 2016;23:163-7. [Crossref] [PubMed]
- Backer CL. Humanitarian congenital heart surgery: template for success. J Thorac Cardiovasc Surg 2014;148:2489-90. [Crossref] [PubMed]
- Balachandran R, Nair SG, Kumar RK. Establishing a pediatric cardiac intensive care unit - Special considerations in a limited resources environment. Ann Pediatr Cardiol 2010;3:40-9. [Crossref] [PubMed]
- Pezzella AT. International cardiac surgery: a global perspective. Semin Thorac Cardiovasc Surg 2002;14:298-320. [Crossref] [PubMed]
- STS Congenital Heart Surgery Data Summary, All Patients, STS Period Ending 12/31/2018. Primary procedure, 35 Most Frequent for All Patients, Last 4 Years (Jan 2015 - Dec 2018). Available online: https://www.sts.org/sites/default/files/Congenital-STSExecSummary_AllPatients.pdf, retrieved 20 April 2021.
- Broberg CS, Shen I, Menashe V, et al. Emergence of the arterial switch procedure for transposition of the great arteries and the potential cost of surgical innovation. J Thorac Cardiovasc Surg 2017;154:1047-51. [Crossref] [PubMed]
- Senning A. Surgical correction of transposition of the great vessels. Surgery 1959;45:966-80. [PubMed]
- Mustard WT, Keith JD, Trusler GA, et al. The surgical management of transposition of the great vessels. J Thorac Cardiovasc Surg 1964;48:953-8. [Crossref] [PubMed]
- Planche C, Lacour-Gayet F, Serraf A. Arterial switch. Pediatr Cardiol 1998;19:297-307. [Crossref] [PubMed]
- Quaegebeur JM, Rohmer J, Ottenkamp J, et al. The arterial switch operation. An eight-year experience. J Thorac Cardiovasc Surg 1986;92:361-84. [Crossref] [PubMed]
- Blume ED, Altmann K, Mayer JE, et al. Evolution of risk factors influencing early mortality of the arterial switch operation. J Am Coll Cardiol 1999;33:1702-9. [Crossref] [PubMed]
- Kiener A, Kelleman M, McCracken C, et al. Long-Term Survival After Arterial Versus Atrial Switch in d-Transposition of the Great Arteries. Ann Thorac Surg 2018;106:1827-33. [Crossref] [PubMed]
- Wernovsky G, Mayer JE Jr, Jonas RA, et al. Factors influencing early and late outcome of the arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg 1995;109:289-301; discussion 301-2. [Crossref] [PubMed]
- Reddy VM, Liddicoat JR, Hanley FL. Midline one-stage complete unifocalization and repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. J Thorac Cardiovasc Surg 1995;109:832-44; discussion 844-5. [Crossref] [PubMed]
- Tweddell J, O'Donnell A. Repair of Taussig-Bing Anomaly, D-Malposed Great Arteries, and Hypoplastic Aortic Arch. CTSNet, November 2019. doi:
10.25373/ctsnet.10069967 . - Hoxha S, Abbasciano RG, Sandrini C, et al. Selective Cerebro-Myocardial Perfusion in Complex Neonatal Aortic Arch Pathology: Midterm Results. Artif Organs 2018;42:457-63. [Crossref] [PubMed]
- Ohye RG, Schranz D, D'Udekem Y. Current Therapy for Hypoplastic Left Heart Syndrome and Related Single Ventricle Lesions. Circulation 2016;134:1265-79. [Crossref] [PubMed]
- Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med 2010;362:1980-92. [Crossref] [PubMed]
- Prabhu NK, Turek JW, Andersen ND. Sustained Total All-Region (STAR) perfusion for Norwood reconstruction with complex intracardiac repair. Perfusion 2021;36:532-4. [Crossref] [PubMed]
- Lisanti AJ, Vittner D, Medoff-Cooper B, et al. Individualized Family-Centered Developmental Care: An Essential Model to Address the Unique Needs of Infants With Congenital Heart Disease. J Cardiovasc Nurs 2019;34:85-93. [Crossref] [PubMed]
- Michielon G, DiSalvo G, Fraisse A, et al. In-hospital interstage improves interstage survival after the Norwood stage 1 operation. Eur J Cardiothorac Surg 2020;57:1113-21. [Crossref] [PubMed]
- Galantowicz M, Cheatham JP, Phillips A, et al. Hybrid approach for hypoplastic left heart syndrome: intermediate results after the learning curve. Ann Thorac Surg 2008;85:2063-70; discussion 2070-1. [Crossref] [PubMed]
- Martin BJ, De Villiers Jonker I, Joffe AR, et al. Hypoplastic Left Heart Syndrome is not Associated with Worse Clinical or Neurodevelopmental Outcomes Than Other Cardiac Pathologies After the Norwood-Sano Operation. Pediatr Cardiol 2017;38:922-31. [Crossref] [PubMed]
- Kiraly L, Shipton S, Tamas Cs, Du Plessis J. Unfavorable growth of the pulmonary vasculature warrants early progression to comprehensive stage-2 operation following hybrid stage-1 palliation for univentricular physiologies with systemic obstruction. 3rd Scientific Meeting of the World Society for Pediatric and Congenital Heart Surgery. Published abstract. WSPCHS Congress Papers 2012;O-8:49.
- Jonas RA. Choosing the right biomaterial. In: Jonas RA, editor. Comprehensive surgical management of congenital heart disease. 2nd Ed. Boca Raton: CRC Press; 2014:247-266.
- Statistics Centre of Abu Dhabi: Health statistics 2010. Available online: http://www.scad.ae/SCAD%20Publications/Health%20, retrieved on 15 November 2012.
- Hamamy H, Antonarakis SE, Cavalli-Sforza L, et al. Consanguineous marriages, pearls and perils: Geneva International Consanguinity Workshop Report. Genet Med 2011;13:841-7.
- CIA Factbook, 2015: UAE statistics. (2015). Available online: https://www.cia.gov/the-world-factbook/countries/united-arab-emirates/#people-and-society, retrieved 20 April 2021.
- Hessini L. Abortion and Islam: policies and practice in the Middle East and North Africa. Reprod Health Matters 2007;15:75-84. [Crossref] [PubMed]
- Bundey S, Alam H, Kaur A, et al. Race, consanguinity and social features in Birmingham babies: a basis for prospective study. J Epidemiol Community Health 1990;44:130-5. [Crossref] [PubMed]
- Blair I, Sharif AA. Population structure and the burden of disease in the United Arab Emirates. J Epidemiol Glob Health 2012;2:61-71. [Crossref] [PubMed]
- Jacobs JP, Jacobs ML, Austin EH 3rd, et al. Quality measures for congenital and pediatric cardiac surgery. World J Pediatr Congenit Heart Surg 2012;3:32-47. [Crossref] [PubMed]
- Van Assen M, Van Den Berg G, Pietersma P. Key Management Models. Pearson Education Ltd, Harlow, 2003.
- Senge PM. The Fifth Discipline. The art and practice of the learning organization. Random House, London, 1990.
- Pezzella AT. Open heart surgery in a developing country. Asian Cardiovasc Thorac Ann 2006;14:355-6. [Crossref] [PubMed]
- Walton M, Deming WE. The Deming management method. Dodd, New York, 1986.
- UAE Ministry of Health. Policy Statement #9 to avert life-threatening emergencies. Page 2 and Schedule 1: Eligibility for Coverage #4. 2008. Available online: http://www.moh.gov.ae/en/OpenData/Pages/OpenData.aspx?Category=All%20Open%20Data, retrieved on 30 August 2014.
- STS Congenital Heart Surgery Database: Available online: https://link.springer.com/article/10.1007/s00246-009-9496-0, retrieved 20 April 2021.
- National Institute for Cardiovascular Outcomes Research, 2014-2017. Available online: https://nicor5.nicor.org.uk/CHD/an_paeds.nsf/vwContent/NCHDA%20Report%20Analyses%202014-17?Opendocument, retrieved 20 April 2021.
- Society of Thoracic Surgeons Congenital Heart Surgery Database, 2012-2018. Available online: http://www.phc4.org/reports/cabg/pediatric/15/, retrieved on 20 August 2020.
- Király L, Tamás C. Primary, single-stage arterial switch operations at a newly-established, comprehensive congenital cardiac center performed in the neonatal age and beyond. Orv Hetil 2015;156:1014-9. [PubMed]
- Dearani JA, Stephens EH, Guleserian KJ, et al. COVID-19: FAQs-Congenital Heart Surgery Recovery and Defining a "New Normal". World J Pediatr Congenit Heart Surg 2020;11:548-56. [Crossref] [PubMed]
- Lytzen R, Vejlstrup N, Bjerre J, et al. Live-Born Major Congenital Heart Disease in Denmark: Incidence, Detection Rate, and Termination of Pregnancy Rate From 1996 to 2013. JAMA Cardiol 2018;3:829-37. [Crossref] [PubMed]
- Cooper DK. A brief history of cross-species organ transplantation. Proc (Bayl Univ Med Cent) 2012;25:49-57. [Crossref] [PubMed]
- Syedain ZH, Haynie B, Johnson SL, et al. Pediatric tri-tube valved conduits made from fibroblast-produced extracellular matrix evaluated over 52 weeks in growing lambs. Sci Transl Med 2021;13:eabb7225. [Crossref] [PubMed]
Cite this article as: Kiraly L. Current outcomes and future trends in paediatric and congenital cardiac surgery: a narrative review. Pediatr Med 2022;5:35.

