Intracranial germ cell tumors in pediatric and adolescent patients in China: systematic review and meta-analysis
Introduction
Low- and middle-income countries bear 80% of the global cancer incidence and face unique challenges in coping with this disease. China has a pediatric population of 271 million, and approximately 45,000 new childhood cancers are diagnosed each year (1), thousands of which are central nervous system tumors. Despite great improvement achieved in acute lymphoblastic leukemia and some solid tumor in China, central nervous system tumors have long been ignored, with their unique challenges remaining unresolved.
Intracranial germ cell tumors (IGCT) are rare neoplasms occurring predominantly in pediatric and adolescent patients and which can be classified histologically into germinomas and non-germinomatous germ cell tumors (NGGCTs, including teratoma, choriocarcinoma, yolk sac tumor, and embryonal carcinoma). They are rare in western countries, but more common in East Asia. Among brain tumor patients under the age of 20, international data shows an overall incidence of 3.9% in the USA and 16.9% (population-based) in Japan (2,3). Our data from several large centers suggest that, among brain tumor patients under the age of 18 in China, 7.27–21.1% (hospital-based) are diagnosed as IGCT (4-9).
Few articles have been published concerning the treatment of IGCT in China, especially for children. Therefore, we conducted this review and meta-analysis of existing evidence to evaluate the treatment condition and prognosis of IGCT in China and to explore the country’s unique problems and challenges in this area.
Diagnostic methods of IGCT vary with countries and years. Some countries rely on surgical pathology for diagnosis, often with a gross total resection. In other countries, germ-cell tumors are not diagnosed with surgery but on the basis of select tumor markers. An increase in alpha-fetoprotein or human chorionic gonadotropin (HCG) to greater than a defined threshold in serum or cerebrospinal fluid can confirm the diagnosis of a secreting NGGCT. However, marker thresholds vary across countries. Before 2015, some hospitals in China preferred radiosensitivity testing (trial RT) or chemosensitivity testing without biopsy to make a germinoma diagnosis for marker-negative patients. In one study, patients were treated with 20 Gy of RT, and a presumed diagnosis of germinoma was made if a marked response (>80% shrink) was observed on follow-up (10). It was the same case in Korea (11), though according to the 4th International CNS germ cell tumor symposium, surgical biopsy is reserved for patients who are marker-negative (12). Because of the diversity of diagnostic methods in China and the fact that results are difficult to compare, in our study, we selected patients with confirmed pathology results. We present the following article in accordance with the PRISMA reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-32/rc).
Materials and methods
Search strategy
The analysis was carried with a search of the PubMed, Embase, Wanfang Data, and CNKI databases for literature published period until October 2020. The languages were limited to English and Chinese. The search strategy involved both Medical Subject Headings (MeSH) terms and keywords, including germinoma, germ cell tumor, intracranial, central nervous system, brain, and China. All References in articles were scanned to identify all relevant articles. The PubMed search history is available in Table S1.
Inclusion and exclusion criteria
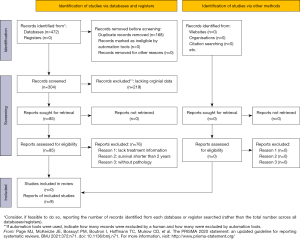
Only clinical studies and case reports in China containing treatment protocols focusing on patients with a pathological diagnosis and a median duration of follow-up of at least 18 months were included in the review. All patients were children or adolescents younger than 24 years. The selection process is illustrated in Figure 1. Data were reviewed by 2 reviewers independently. Disagreement between the reviewers was settled by a third reviewer. From the 472 articles found, 168 articles were removed as duplicates and 219 articles were excluded for lacking original data, being animal or adult studies, or otherwise being considered not relevant according to a reading of the titles and abstracts. Consequently, 85 articles were retrieved for further assessments according to the full text, 70 of which were excluded because they lacked treatment information and 6 of which were excluded for not having confirmation of the pathology. Finally, 9 studies (9,13-20) that met the inclusion criteria were included in the analysis.
Risk of bias assessment
Data related to the study and quality assessment were scrutinized by 2 reviewers independently. Disagreement between the reviewers was settled by a third reviewer. For case series, we used the standardized critical appraisal instrument from the Joanna Briggs Institute (JBI; Table S2).
Statistical analysis
Outcomes of interest were overall survival (OS) and progression-free survival (PFS). Data in the tables, texts, or figures of the original papers were extracted if the outcomes were not reported directly in the original paper. Continuous data are expressed as mean ± SD. The event rates were pooled using the random effects model. The inverse variance method was used to compare the outcomes. All P values were 2-sided, and a P value <0.05 was considered significant. Statistical calculations were performed using R software version 4.0 (The R Foundation for Statistical Computing).
Results
Characteristics of studies
The review is presented in the flow diagram (Figure 1). A total of 9 reports comprising 218 patients were included in the review (Table 1) (9,13-20). There were 75 female and 143 male patients analyzed. Mean age at diagnosis was 10.5±4.6 years (range, 3–24 years). There were 118 germinomas and 100 NGGCTs. The most common tumor site was the suprasellar region (35.3%), followed by the pineal area (26.1%), and other locations (25.0%), including the basal ganglia, thalamus, third ventricle, and cerebellum. In addition, 29 cases were multifocal tumors including 13 cases (6%) that were bifocal tumors (suprasellar and pineal area).
Table 1
| Author, year | Patients (n) | Age [years] | Male (%) | Diagnosis | Study design | Location | Follow-up [median/months] |
|---|---|---|---|---|---|---|---|
| Zhang 2018 | 52 | 10.8 [1–18] | 0.5 | Pathology | Case series | Beijing, multicenter | 30 [8–53] |
| Dong 2019 | 43 | 6.0 [3.6–15.5] | 0.7 | Pathology | Case series | Shanghai, single center | 31.8 [1–144] |
| Zhao 2017 | 16 | 11.1 [3–24] | 0.6 | Pathology | Case series | Beijing (Tsinghua University), single center | 17.5 [4–45] |
| Wanggou 2012 | 29 | 13.0 [6–24] | 0.7 | Pathology | Case series | Changsha, single center | 54 [5–60] |
| Wong 2008 | 6 | 10.9 [9.3–13.6] | 1.0 | Pathology | Case series | Taipei, single center | 143 [73–190] |
| Wang 2018 | 11 | 11.9 [7–16] | 0.8 | Pathology | Case series | Chengdu, single center | 36 [9–72] |
| Tang 2007 | 14 | 12.4 [8–18] | 0.9 | Pathology | Case series | Beijing (Tiantan Hospital), single center | 56 [6–87] |
| Xiao 2011 | 15 | 16.3 [8–24] | 1.0 | Pathology | Case series | Guangzhou, single center | 24 [4–120] |
| Lian 2014 | 32 | 9.5 [6–14] | 0.5 | Pathology | Case series | Beijing (Peking Union Medical College), single center | 60 [4–216] |
For the survival analysis, 6 reports including 97 germinomas were analyzed (9,13-16,18) and 6 reports including 78 NGGCTs were analyzed (13,16-20). For germinoma, the total number of the patients in the radiotherapy (RT)-alone group and the radiotherapy combined with chemotherapy (CMT) group were 38 and 59, respectively. In the CMT group, whole brain (WB), whole ventricle (WV), or craniospinal irradiation (CSI) was applied, with 20–36 Gy was given in WB/WV/CSI and 10–49 Gy was given in tumor-bed boost. The chemotherapy protocol included 2–6 cycles of carboplatin, etoposide, cisplatin and ifosfamide; or vinblastine, bleomycin, cisplatin and etoposide. In the RT-only group, focal irradiation and/or CSI/WB/WV was applied with the radiation dose ranging from 40 to 50 Gy in focal irradiation and 20 to 38 Gy in WB/CSI/WV.
For NGGCTs, 78 patients were included. Some patients received chemotherapy combined with surgery and others received chemotherapy, radiation, and surgery. The chemotherapy protocol included 2–6 cycles of etoposide, cisplatin and ifosfamide. The radiation strategy included local irradiation, WB radiotherapy, WV radiotherapy, CSI, and local radiation plus WV/WB/CSI. The radiation dose ranged from 29 to 55 Gy in focal irradiation and from 17.8 to 39.6 Gy in WB/CSI/WV. Some studies (13,17,18,20) did not describe the radiation doses.
Meta-analysis
The main results of this meta-analysis are shown in Figures 2-4 and summarized in Table 2. According to the data available, we collected the 3-year PFS rate and OS rate.
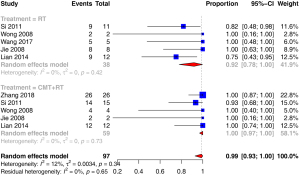
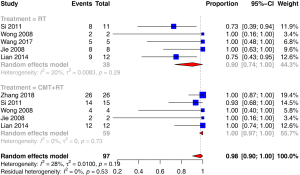

Table 2
| Treatment | Outcome | 95% CI | I2 | Heterogeneity P value | Difference between groups P value | |
|---|---|---|---|---|---|---|
| Germinoma | ||||||
| 3-year PFS | ANY | 0.98 | (0.9, 1) | 28% | 0.19 | |
| RT | 0.9 | (0.74, 1) | 20% | 0.29 | 0.0415 | |
| CMT + RT | 1 | (0.97, 1) | 0% | 0.73 | ||
| 3-year OS | ANY | 0.99 | (0.93, 1) | 12% | 0.34 | |
| RT | 0.92 | (0.78, 1) | 0% | 0.42 | 0.0396 | |
| CMT + RT | 1 | (0.97, 1) | 0% | 0.73 | ||
| NGGCT | ||||||
| 3-year PFS | ANY | 0.68 | (0.51–0.83) | 40% | 0.14 |
PFS, progression free survival; ANY, any treatment including radiotherapy and chemotherapy, including all the patients; RT, radiotherapy; CMT, radiotherapy combined with chemotherapy; OS, overall survival; NGGCT, non-germinomatous germ cell tumor.
The pooled 3-year PFS and OS for germinoma revealed by a random effects model of the CMT + RT group was higher than that of the RT-only group (100% vs. 90%, P=0.0415; 100% vs. 92%, P=0.0396). In the included studies, the pooled 3-year PFS of germinoma was 98% while that of NGGCT was 68%.
Discussion
IGCTs include germinomas and NGGCTs. Intracranial germinomas account for two-thirds of IGCTs. Radiotherapy including craniospinal RT was once the standard treatment for intracranial germinoma, and the cure rate for RT monotherapy exceeds 90%. Because of the severe side effects, such as neurocognitive impairment and endocrinopathies, in some centers chemotherapy combined with radiotherapy has been attempted to enable reduction in both RT dose and volume. The consensus on IGCTs in 2015 pointed that aim in germinoma treatment is to maintain an excellent OS rate while attempting to minimize late effects. It was further suggested that radiotherapy include focal fields and at least the ventricles, and chemotherapy is an effective strategy to reduce the dose of radiotherapy for localized germinomas (12). In our meta-analysis, both the RT and chemotherapy combined groups gained satisfying outcomes, while the chemotherapy combined groups achieved better survival. The 3-year PFS and OS for germinoma were 99% and 98%, respectively. The SMC-G13 trial demonstrated that induction chemotherapy reduces radiation therapy dose and volume in intracranial germinomas (21). Patients received 4 cycles of chemotherapy with carboplatin/etoposide alternating with cyclophosphamide/etoposide. A dose of 18 Gy of craniospinal RT for metastatic tumors, whole ventricle RT for localized tumors followed by 12.6 Gy boost RT to the primary tumor bed was administered after chemotherapy. The 5-year PFS and OS was 96.7% and 96.2%, respectively. Therefore, we suggest that for patients with pure germinomas, chemotherapy combined therapy would be a better choice to enable reduction in both RT dose and volume.
Patients with NGGCT had worse outcomes compared with those with germinoma. The 5-year OS in patients with NGGCT treated with radiation alone or chemotherapy alone ranged from 20% to 40% (22). For patients with IGCT containing malignant non-germinomatous components, the consensus points out that a combination of chemotherapy and RT be applied to maximize the chance of cure (12). In our study, the 3-year PFS for NGGCT was 68%, but the data were not convincing due to the considerable heterogeneity in chemotherapy doses and volumes. The recent international COG ACNS0122 trial and SIOP CNS GCT 96 trial have reported encouraging results. The COG ACNS0122 trial used 6 cycles of chemotherapy of 36 Gy CSI and 54 Gy to the primary tumor bed (23). The 5-year PFS and OS were 84% and 93%, respectively. The SIOP trial 96 used 4 cycles of chemotherapy for those with localized disease, with a 54 Gy dose being administered to the involved field (24). The 5-year PFS and OS were 72% and 82%, respectively.
Although there are many patients (at least 531 according to the populations reported) diagnosed with IGCT every year, (218/20 years) few patients receive proper treatment in China. Many unique challenges exist in the pediatric neuro-oncology service in China. Lack of well-trained pediatric neuro-oncologists, lack of multidisciplinary care teams, high rates of midtreatment abandonment, lack of adequate knowledge of IGCTs, and lack of children’s hospitals for pediatric patients with central nervous system tumors (without basic RT facilities in any children’s hospital in China) remain the key challenges in China (1). This is frustrating for patients and their families who may receive a confusing array of opinions, leading to delays for adjuvant chemotherapy or radiation after surgery, or even abandonment. Even in Shanghai, before 2017, due to a lack of well-trained pediatric neuro-oncologists, most pediatric central nervous system tumor patients were admitted to adult hospitals. Since October 2017, after cooperation began with St. Jude Children’s Research Hospital, Shanghai Children’s Medical Center began receiving children with central nervous system tumors. We began receiving IGCT patients in October 2018. The COG’s ACNS1123 protocol was adopted in our center. Thus far, we have received 105 children with brain tumors, with 40 of these cases being IGCTs: 7 were germinomas, and the other 33 were NGGCTs. Of these 40 patients, 10 were girls and 30 were boys, with the endian age at diagnosis being 10 years (range, 2 months to 16 years). For germinoma, 4 patients received RT alone after surgery, and another 3 patients received 4 cycles of chemotherapy with carboplatin and etoposide before radiation (24 Gy WV and 16 Gy local tumor boost). All 7 patients remained in complete remission after a median follow-up of 12 months (range, 2–25 months). For NGGCTs, 33 patients received 6 cycles of chemotherapy with carboplatin and etoposide being alternated with ifosfamide and etoposide. After chemotherapy, the patient was evaluated to determine whether they receive radiation (36 Gy CSI and 54 Gy local tumor boost), second surgery, or autologous stem cell transplantation. One patient abandoned treatment halfway, 2 patients relapsed after half a year and 1 year respectively, 14 patients are still receiving treatment, and the remaining 16 patients were surviving after a median follow-up of 15 months (range, 11–24 months).
The deficiencies in treatment in China emphasized the necessity in establishing multidisciplinary care teams in pediatric hospitals receiving children with central nervous system tumors. The treatment of pediatric and adolescent IGCTs requires close collaboration with interdisciplinary teams consisting of neurosurgeons, radiologists, pathologists, radiation oncologists, and pediatric neuro-oncologists. And a national treatment protocol/study would also be useful to standardize treatment within China. Great effort needs to be made in the future to improve IGCT outcomes.
Some limitations to our analysis should be mentioned. First, studies included were few in number and noncomparable, and the I2 statistics indicated a high publication bias, which weakens the evidence based on the results drawn. Second, across the different groups, the choice of treatment strategy varied, including the radiation dose and fields, as well as the chemotherapy drugs and doses, which increased the heterogeneity of the pooled outcomes and limited the validity of the conclusion. Third, other limitations include level of tumor markers, e.g., AFP <1,000 vs. >1,000 ng/mL; response to chemotherapy before radiotherapy; residual lesion with/without 2nd look surgery, which we did not mentioned in our context.
Overall, greater effort is needed in the future to improve survival in children with IGCTs, and multidisciplinary input is key to achieving satisfactory outcomes.
Acknowledgments
We would like to thank Yi Le and Yali Han for collecting the data.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ibrahim Qaddoumi, Anthony Liu and Chenchen Sun) for the series “Pediatric CNS Tumors in China” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-32/rc
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-32/prf
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-32/coif). The series “Pediatric CNS Tumors in China” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pak-Yin Liu A, Moreira DC, Sun C, et al. Challenges and opportunities for managing pediatric central nervous system tumors in China. Pediatr Investig 2020;4:211-7. [Crossref] [PubMed]
- Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol 2015;17:iv1-62. [Crossref] [PubMed]
- Takami H, Fukuoka K, Fukushima S, et al. Integrated clinical, histopathological, and molecular data analysis of 190 central nervous system germ cell tumors from the iGCT Consortium. Neuro Oncol 2019;21:1565-77. [Crossref] [PubMed]
- Gui T, Zhang QH, Zhang TG, et al. Central nervous system tumors in childhood: analyses of 330 cases. Chinese Journal of Clinical and Experimental Pathology. 2008;24:403-6.
- Zhang R, Shen WQ, Zhou LF. Primary pediatric central nervous system tumors statistic: study of 763 cases in a single institution. Zhonghua Yi Xue Za Zhi 2007;87:442-7. [PubMed]
- Zhu T, Yu YH, Zhang DJ, et al. Clinical analysis of central nervous system tumors in children: a report of 468 cases. Chinese Journal of neurosurgery 2012;28:8-12.
- Wang J, Zhao YY, Yang JY, et al. Clinal analysis of pediatric central neuvous system tumors: a retrospctive study of 219 cases. Guangdong Medical Journal. 2015;36:2621-4.
- Li JZ, Chu H, Miao N, et al. Pediatric tumors of nervous system tumors: a clinicopathologic study of 398 cases. Chinese Journal of diagnostic Pathology. 2019;26:333-7.
- Wong TT, Chen YW, Guo WY, et al. Germinoma involving the basal ganglia in children. Childs Nerv Syst 2008;24:71-8. [Crossref] [PubMed]
- Gao JM, Zhang Y, Zheng MM. Long-term results of radiotherapy for intracranial germinomas. Shanxi Medical Journal. 2006;35:546-8.
- Byun HK, Yoon HI, Cho J, et al. Optimization of Intracranial Germinoma Treatment: Radiotherapy Alone with Reduced Volume and Dose. Int J Radiat Oncol Biol Phys 2020;108:657-66. [Crossref] [PubMed]
- Murray MJ, Bartels U, Nishikawa R, et al. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol 2015;16:e470-7. [Crossref] [PubMed]
- Wang M, Zhou P, Zhang S, et al. Clinical features, radiologic findings, and treatment of pediatric germ cell tumors involving the basal ganglia and thalamus: a retrospective series of 15 cases at a single center. Childs Nerv Syst 2018;34:423-30. [Crossref] [PubMed]
- Wanggou S, Jiang X, Yuan X, et al. Prognostic value of OCT4 in primary intracranial germinoma: a single institute analysis of 31 cases. Br J Neurosurg 2012;26:237-46. [Crossref] [PubMed]
- Tang J, Ma Z, Luo S, et al. The germinomas arising from the basal ganglia and thalamus. Childs Nerv Syst 2008;24:303-6. [Crossref] [PubMed]
- Lian X. A retrospective study of suprasellar tumor-like lesions and intracranial germinomas. [Master]: Peking Union Medical College; 2014.
- Xiao G. Clinic and pathology characteristics research of pineal region mixed germ cell tumor. [Master]: Southern Medical University; 2011.
- Zhang LJ, Zhao XJ, Xiao J, et al. Diagnosis, treatment and follow-up of 52 cases of primary intracranial germ cell tumors diagnosed by pathology under the multidisciplinary collaboration. Journal of China Pediatric Blood and Cancer 2018;23:178-83.
- Dong XS, Yang J, Han YF, et al. The treatment and prognosis of primary pediatric intracranial nongerminomatous malignant germ cell tumors. Chinese Journal of Neurosurgery 2019;35:776-81.
- Zhao YP, Zhang YQ, Duan HY, et al. Intracranial mixed germ cell tumor. Zhonghua Yi Xue Za Zhi 2017;97:661-5. [PubMed]
- Lee JW, Lim DH, Sung KW, et al. Induction Chemotherapy Reduces Radiation Therapy Dose and Volume in the Treatment of Intracranial Germinoma: Results of the SMC-G13 Trial. Int J Radiat Oncol Biol Phys 2020;108:649-56. [Crossref] [PubMed]
- Fangusaro J, Wu S, MacDonald S, et al. Phase II Trial of Response-Based Radiation Therapy for Patients With Localized CNS Nongerminomatous Germ Cell Tumors: A Children's Oncology Group Study. J Clin Oncol 2019;37:3283-90. [Crossref] [PubMed]
- Goldman S, Bouffet E, Fisher PG, et al. Phase II Trial Assessing the Ability of Neoadjuvant Chemotherapy With or Without Second-Look Surgery to Eliminate Measurable Disease for Nongerminomatous Germ Cell Tumors: A Children's Oncology Group Study. J Clin Oncol 2015;33:2464-71. [Crossref] [PubMed]
- Calaminus G, Frappaz D, Kortmann RD, et al. Outcome of patients with intracranial non-germinomatous germ cell tumors-lessons from the SIOP-CNS-GCT-96 trial. Neuro Oncol 2017;19:1661-72. [Crossref] [PubMed]
Cite this article as: Zhang A, Gao Y. Intracranial germ cell tumors in pediatric and adolescent patients in China: systematic review and meta-analysis. Pediatr Med 2022;5:36.