A narrative review of pitfall and progress in management of inflammatory bowel disease in children
Introduction
Inflammatory bowel diseases (IBDs) broadly classified as ulcerative colitis (UC) and Crohn’s disease (CD), are progressive chronic intestinal disorders commonly diagnosed in the pediatric age group (1). They are widely believed to result from aberrant host immune response to environmental triggers such as dietary factors and gut microbiota in genetically susceptible individuals (2,3). The incidence of IBD is lower in Asia, but a more severe IBD phenotype is observed in the Asian population in comparison to the western world (4). Emerging epidemic of IBD across Asia is unique, it is driven by different sets of genetic and environmental risk factors, compared to the Western population (5,6). The diagnosis of IBD poses additional challenges in Asia, due to a higher prevalence of intestinal tuberculosis (TB) and resource limitations (7).
IBD is common enough to fall within the caseload of family physicians and pediatricians. Whilst the majority of children with IBD, present with classic symptoms of weight loss, abdominal pain and diarrhea, some may present with growth failure and anemia with no or minimal symptoms, particularly those with isolated small bowel CD. These children miss early diagnoses and are at greater risk of complications, such as fistulae and strictures (8). IBD in children also runs a more aggressive course compared to adults, as children face years of severe disabling symptoms and a high risk of bowel surgery and cancer. The purpose of this narrative review is to provide a contemporary overview of the IBD in children with a focus on pitfalls, challenges and progress, drawing upon up-to-date evidence.
As the topic of IBD in children is very broad, authors provide readers with a critical and objective review of current knowledge in this area. While a systematic literature review is beyond the scope of this narrative review, every attention is given to correct unbiased interpretation of data and citation of peer-reviewed articles, sourced from PubMed, Google Scholar and EMBASE databases (Table 1). I present the following article in accordance with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-11/rc).
Table 1
| Items | Specification |
|---|---|
| Date of search | December 2020 |
| Databases and other sources searched | PubMed, Google Scholar, Medline |
| Search terms used | Crohn’s disease, ulcerative colitis, mucosal healing, treatment targets |
| Timeframe | None |
| Inclusion and exclusion criteria | English language |
| Selection process | Author selected peer reviewed articles based on citation, and relevance to paediatric age group |
IBD is a spectrum
Traditionally, IBD is classified as CD, UC or IBD-unclassified (IBD-U) in those with overlapping features of CD and UC. However, IBD is a heterogeneous group of chronic intestinal inflammatory disorders that can be diagnosed after careful exclusions of infections and immunologic disorders (Table 2) (9,10).
Table 2
| Infections | Non-infectious |
|---|---|
| Yersinia | Sarcoidosis |
| Salmonella | Behcet disease |
| TB | Hermansky-Pudlak |
| Entamoeba histolytica | Wiskott-Aldrich syndrome |
| Histoplasmosis | Common variable immuno-deficiency |
| Coccidioides | Chronic granulomatous disease |
| C. difficile | IL-10 receptor defects |
| X-linked lymphoproliferative disease | |
| Immune dysregulation-polyendocrinopathy-enteropathy-X-linked |
IBD, inflammatory bowel disease; TB, tuberculosis.
Diagnosis of IBD in children
The IBD is usually suspected on combination of symptoms, examination, and abnormal laboratory screening tests. Chronic abdominal pain, diarrhea and weight loss are commonly seen in CD, but almost 25% of children may manifest non-specific symptoms such as tiredness, delay in linear growth and pubertal failure. On the other hand, bloody diarrhea is the most common manifestation of UC. Extra-intestinal manifestations of IBD including arthralgia, aphthous ulcers fever and erythema nodosum. They are seen in almost 25% of children at diagnosis and when present, contribute to the increased disease severity (Table 3).
Table 3
| Symptoms and signs | CD | UC |
|---|---|---|
| Weight loss | ++++ | ++ |
| Abdominal pain | ++++ | ++ |
| Growth failure | +++ | + |
| Perianal disease | ++ | – |
| Mouth ulcers | ++ | + |
| Fever | ++ | + |
| Diarrhoea | ++ | ++++ |
| Rectal bleeding | ++ | ++++ |
| Erythema nodosum | + | + |
| Anemia | +++ | +++ |
| Arthritis | + | + |
+, seen; ++, moderate; +++, frequent; ++++, very frequent; –, not seen. IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis.
It is important to consider IBD in children with chronic abdominal pain but it should be remembered that it is frequently encountered in other common gastrointestinal (GI) conditions, such as functional abdominal pain, post-infectious irritable bowel syndrome, and coeliac disease in children. Non-voluntary weight loss is an important red-flag sign of serious organic pathology, a detailed record of weight, height, growth velocity and puberty are vital for diagnosis. In those with a strong family history of IBD, weight loss a high index of suspicion should be maintained and laboratory tests should be ordered for screening purposes. The presence of anemia, elevated serum inflammatory markers including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) are supportive for a diagnosis of IBD in children presenting with symptoms of IBD, however, normal laboratory findings may be seen in 21–50% of children with IBD (11). Limitations of these serum-based inflammatory markers can be overcome by utilising fecal biomarkers, such as fecal calprotectin (FCP) as it possesses a very high negative predictive value, for inflammatory intestinal disease. FCP is predominantly a neutrophilic protein that can be measured in stools, it is a highly accurate non-invasive test that differentiates between functional and inflammatory bowel disorder, with a normal value <50 mcgm/gm of stool. Although it may be a cost-effective screening strategy when in doubt, it should be noted that elevated levels of FCP can be seen in cases with infectious colitis, juvenile polyps, NSAIDS exposure and in healthy infants (12,13).
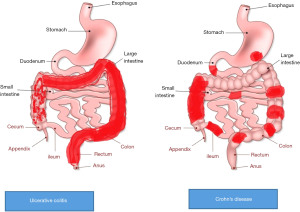
All children with clinical suspicion of IBD require a complete work up including upper GI endoscopy and ileo-colonoscopy with multiple biopsies and a cross sectional imaging. There are typical endoscopic features that help to differentiate CD with UC. In a child with classic UC, a continuous colonic mucosal inflammation, usually starting from the rectum, without small intestine involvement is common, although less common features include gastritis, backwash ileitis or relative rectal sparing.
On the other hand, a classic CD is non-continuous aphthous or linear ulcers, involving any part of the GI tract (Figure 1). UGI tract inflammation was previously considered a specific finding of CD but it can also be seen in children with UC, although serpiginous gastric or duodenal ulcers would favour a diagnosis of CD (14). CD can also present atypically with continuous superficial inflammation involving the colon, isolated oral or perianal disease without any bowel involvement. In those with atypical findings on endoscopy, with overlapping features of CD and UC, histology and small bowel imaging, can be helpful for more accurate classification. Histological findings such as lymphocytic esophagitis, focal enhanced gastritis, epithelioid granulomas not associated with ruptured crypt favour a diagnosis of CD over UC. On the other hand, histological features can make differentiation difficult between colonic CD and UC difficult, such as rectal sparing on endoscopy, isolated non-serpiginous gastric ulcers, transmural inflammation in acute severe colitis (14).
Small bowel imaging preferably MR enterography over CT enterography to minimize radiation exposure should be performed at diagnosis in all the adolescents with IBD, except in those with definitive UC, for accurate classification of disease extent and rule out complicating CD (strictures and fistulising disease) (15).
IBD in children is more aggressive and a distinctive disease than adult-onset IBD
Cohort studies comparing the natural history of pediatric-onset vs. adult-onset IBD (CD and UC), confirms its more aggressive nature, with extensive anatomical involvement, rapid progression, and increased disease activity, despite greater immunosuppression use in children with IBD compared to adults (16-18). Not only the disease phenotype in children is aggressive, but there are also distinctive issues in children with IBD such as linear growth failure, delayed puberty, poor bone density and psychosocial impact of a chronic uncurable illness.
Delay in linear growth usually precedes the onset of GI symptoms with reported 5–8 cm loss of final adult height, despite optimizing treatments (19). Delay in diagnosis in patients with IBD should be avoided at all costs, as it is associated with complicated disease behaviour with and higher risk of surgery and linear growth loss (11,20). The expanding management paradigms for treatment of CD have not changed the prevalence of linear growth failure although recent reports utilizing more an earlier intensive treatment approaches using anti-TNF agents promote linear growth possibly by more effective control of inflammation and minimizing exposure to chronic steroid therapy (21). Intestinal TB is the more frequently encountered in Asia compared to CD leading to widespread use of empirical anti-tubercular treatments leading to delay in diagnosis and complications such as stenosis, requiring intestinal (22). Taken together increasing incidence of IBD in Asia, poor awareness, resource limitations and a higher prevalence of common IBD mimics, such as TB and amoebiasis are unique challenges facing pediatricians in Asia.
Treatment options and goals of IBD management
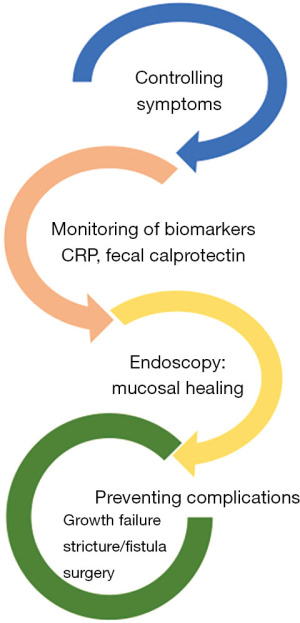
It is important to conceptualize goals of treatment as immediate, medium and long term. While the immediate goals following a diagnosis are controlling symptoms and maximising quality of life. Medium-term goals are tighter control of disease, striving for deeper healing of the bowel by strict monitoring using surrogate biomarkers such as CRP and FCP to make treatment adjustments. Ultimately the long-term goals are to avoid complications such as growth failure, stricture, fistulae, surgery and hospitalization (Figure 2). Striving to achieve these goals have the potential to prevent complications related to disease while minimizing adverse effects of the medications such as corticosteroids (CS), that are not associated with deep remission (23).
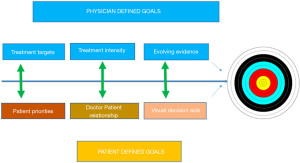
It is equally important to remember that while objective control of the disease is important for the best long-term outcomes, patients focus is usually on immediate intensity of their physical symptoms. There are also important considerations in management, such as psychological distress of a new diagnosis and legitimate concerns of serious side effects from life-long usen of immunosuppressing medications. One of the major challenges in the treatment of IBD is that the intensity of symptoms does not always correlate well with the objectively measured inflammatory burden based on laboratory, endoscopy, or imaging. This is an important consideration for determining treatment choices, our patients need to be educated that poorly controlled inflammation despite absence of symptoms may lead to disabling long-term outcomes (24,25). An appropriate treatment decision involves careful consideration of multiple factors including the disease severity, presence of risk factors associated with disease progression, and the risk and benefits of proposed treatments. Recent studies have examined clinical, genetic and serological risk factors associated with complicating CD in children, defined as stricturing or fistulising CD and/or those requiring intestinal resection in CD. Clinical parameters at diagnosis such as age <14 years, ethnicity, impaired linear growth, elevated anti-Saccharomyces cerevisiae (ASCA) and anti-flagellin (anti-CBir) serology and early non-response to treatments were noted as poor outcome predictors in CD (26-29). The predictors of poor outcomes in UC were; involvement of the entire colon, severe symptoms at diagnosis and again poor early response to treatment (30-36).
It is equally important to know predictors of good outcomes in IBD. Multiple studies confirm that early mucosal healing (MH) is associated with good outcomes. MH, defined as the absence of ulceration in CD and UC, has therefore emerged as an important treatment endpoint. When achieved and maintained, it leads to a reduction in relapse, complications, hospitalization and surgical resection in both CD and UC (23,37,38).
While the concept of MH is intuitive and important but it is not a novel concept. Blood pressure targets in chronic hypertension and glycosylated hemoglobin in diabetes have been around for decades reinforcing the idea that tight control of chronic disease, prevents end-organ damage (Figure 2). However, demonstrating MH as a key target is still an invasive strategy that requires repeated endoscopy. Using surrogates of MH are therefore critical in monitoring progress. FCP remains one of the most widely available and reliable surrogate biomarkers of MH. Several studies report an FCP cut-off for MH to be under 250 µgms/gm of stool, although a lower value (<100) may reflect deeper healing commonly referred to as microscopic healing in UC or transmural healing in CD (39-41).
The most important progress in the management of IBD has been the appreciation that conventional treatment endpoints relying solely on patient-reported symptoms are outdated as many with significant mucosal ulcerations on endoscopy report no symptoms, yet these patients remain at high risk of progressive intestinal damage. There are limited prospective pediatric studies, however emerging data indicate that confirmation of MH using repeat endoscopy is feasible, and those achieving early healing of the intestinal mucosa have better long-term outcomes at 1, 2 and 3 years in CD (42,43).
Treatment options
Following a thorough diagnostic workup and after considering the severity of activity and risk factors of poor disease course an individualized treatment should be proposed. When making treatment recommendations it is important to consider a patient’s perspective, their own and their caregiver’s concerns about side effects, psychosocial state, and cost. Clinicians often approach treatment choices keeping in mind risk factors of disease progression, such as deep ulcerations on endoscopy, impaired linear growth, perianal fistula, extra-intestinal manifestations, or poor response to treatments.
Treatment options for CD can be divided into fast acting agents, also known as induction therapies, and maintenance therapies First-line induction therapies in pediatric CD include an exclusive 8-week course of liquid-based polymeric diet also known as exclusive enteral nutrition (EEN) therapy or a 2-month weaning course of oral prednisolone starting at 1 mg/kg with a maximum dose of 40 mg. EEN is widely accepted as the first-line therapy for pediatric CD as it has no side effects, comparable efficacy in controlling symptoms to that of CS, and a greater ability to heal intestinal ulcers in addition to nutritional benefits (44,45). The two most common conventional maintenance therapies include immune-modulators such as azathioprine (2–2.5 mg/kg/day) and methotrexate (10–25 mg/m2 weekly). These agents have long latency of action and are primarily used to reduce the risk of relapses, and minimize CS dependency.
Anti-tumour necrosis factor (anti-TNF) agents such as infliximab (IFX) and adalimumab are fast-acting, highly efficacious agents used both for induction and maintenance therapy. Anti-TNF agents are currently used in children with CD after failing an adequate trial of steroids or EEN with or without concurrent use of conventional immune modulators. Anti-TNF should be considered upfront in children with complex perianal fistula, deep colonic ulceration, severe growth delay or in those with stricture or intestinal fistulising CD (44,46,47).
Treatment of UC in children follows a similar step-up approach unless disease is severe at diagnosis. Oral and or topical aminosalicylates (ASA) therapy are preferred in those with mild to moderate disease at diagnosis. CS are used in those with moderate to severe disease or in those with poor or insufficient response to ASA therapy. Prednisolone is used at a starting dose of 1 mg/kg with maximum dose of 40–50 mg. Early CS dependency (inability to wean steroids with relapse in symptoms) or poor response to CS (refractory disease) is not uncommon in children, almost half of the children with moderate to severe UC require additional therapies, either with azathioprine or anti-TNF agents or surgery (48,49).
Therapies for IBD are rapidly evolving and there are many newer agents on the horizon, especially ‘newer biologic agents’ (i.e., antibodies to specific proteins involved in the inflammatory process such as ustekinumab/risankizumab (anti-IL-12/23), vedolizumab (anti-α4β7 integrin) and oral small synthetic molecules such as Janus kinase (JAK) inhibitors (tofacitinib, upadacitinib, filgotinib) and oral sphingosine 1 phosphate 1 receptor (S1P) modulators (ozanimod, etrasimod, fingolimod) (50,51).
Target therapies over the last two decades have revolutionized the management of IBD, newer small molecule drugs such as JAK inhibitors can be given orally, they are less immunogenic and safer compared to injectable biologics including anti-TNF’s, IL-12/23 inhibitors. However as there are multiple cytokine pathways involved in pathogenesis IBD, we are yet to figure out the best right drug fit for our patients.
Monitoring and optimization of therapy
There is emerging support for personalized therapy using pharmacogenomics, pharmacokinetics and pharmacodynamics measures to optimize therapy. Using genetic panels to predict severe bone marrow toxicity in those commencing thiopurines such as thiopurine methyltransferase (TPMT) and Nudix hydrolyse NUDT15 genotype, or presence of HLADQA105 haplotypes that increase risk of antibody formation against anti-TNF examples of some significant advances in personalizing therapies using pharmacogenomics (52,53).
Using trough drug levels of biological agents (pharmacokinetic monitoring) and combining them with surrogate biomarkers of mucosal inflammation such as CRP and FCP (pharmacodynamics monitoring) are redefining how we can treat and manage IBD. For example, in a patient with CD who is on standard 5 mg/kg/dose dosing of IFX reporting GI symptoms suggestive of relapse, a good therapeutic decision would require both pharmacodynamic and pharmacokinetic information. An elevated FCP provides pharmacodynamic information about disease activity and a therapeutic trough level of IFX below <3 µg/mL would suggest insufficient control of a disease that could be related to the pharmacokinetic failure. In these circumstances, an increased dose of IFX is likely to benefit the patient. On the other hand, if the IFX trough levels were above 12 µg/mL it would be considered a pharmacodynamic failure and dose increase is unlikely to benefit the patient.
Emerging data studies suggest early intensive monitoring using surrogates and therapeutic dose optimization following diagnosis are critical for the best long-term outcomes (53,54).
Risk minimization with immune-modulators and biologics treatment
Communicating the risk and benefits of lifelong immune-modulating therapies for pediatric IBD is a very delicate subject, often complicated by families’ strong perceptions, fears and inadequate interpretations of medical data, and the child’s preference for oral or injectable medications. When discussing serious risks and benefits of treatments, a visual frequency display should be used instead of statistical jargon. When discussing the subject of risk and benefits of therapies, it’s important to highlight the risk of poorly controlled disease and the benefits of early effective control of chronic bowel inflammation. For example, when describing the risk of azathioprine causing a more than twofold increase in the risk of lymphoma is far more unsettling than saying the risk increases from 2 in 10,000 to 4 in 10,000.
Poor medication adherence can be circumvented by clearly communicating the benefits of maintenance therapy, even in the absence of symptoms and reminding patients of treatment goals. Shared-care management plans involving physicians with clear documentation of anticipated side effects and phone support can improve surveillance of toxicity, rare infections and malignancy (55,56).
In addition, children with IBD on immune-modulators or biologics (e.g., CS, azathioprine, methotrexate or anti-TNFs) remain at high risk of opportunistic infections; therefore, it is vital to minimize this risk by optimizing live vaccination at diagnosis or 2 months before commencing immune-modulators or biologics (56,57). SARS-CoV-2 infection risk in patients with IBD is comparable to the general population. Outcomes of COVID-19-positive IBD patients are worse on steroids or 5-ASA but outcomes are better with biological agents (58).
Vaccination records, travel history and exposure to TB, should be checked before commencing biologics. All children newly diagnosed with IBD should have vaccination titers tested for hepatitis B and varicella. If the immunity to hepatitis B and varicella cannot be established, the child should have their vaccination schedule updated as soon as possible while on non-immunosuppressive treatments, such as EEN in CD and 5-ASA in UC. Quantiferon for TB or tuberculin skin test should be performed before commencing immunomodulators and biologics due to higher risk of TB reactivation on anti-TNF’s on all children with IBD regardless of history of non-exposure to TB. Once the child has started immunosuppressive agents and/or biologics, the use of live vaccinations [e.g., measles, mumps and rubella (MMR), varicella, yellow fever, bacillus Calmette-Guérin (BCG)] is contraindicated and the response to non-live vaccinations, such as hepatitis B can attenuate (57).
Ongoing interval monitoring of full blood counts and liver function tests, to detect features of bone marrow suppression and hepatotoxicity needs to continue with the use of immune modulators. Azathioprine-related bone marrow suppression is a serious adverse reaction reported in 2% of patients (59). It can occur very abruptly at any time during the treatment (first few weeks to years), but much higher rates are seen during the first 3 months of therapy. Symptoms may be absent or upper respiratory tract infection (URTI)-like, but severe pancytopenia can lead to sepsis and death. Moderate neutropenia often responds to drug withdrawal. Thiopurine metabolite testing helps to optimize the use and dosing of thiopurine using 6 thioguanine (TG) levels and determine patients that are shunting towards the unfavourable hepatotoxic metabolite [methyl-mercaptopurine (MMP)]. In addition, low levels of both metabolites, TG and MMP can also identify non-compliant patients (60).
Similarly, the presence and concentration of biologic agents and anti-drug antibodies more commonly anti-TNFs (anti-IFX, anti-adalimumab) antibodies can be regularly monitored. There are published recommended target trough levels to enable maximal therapeutic benefits. Therapeutic anti-TNF drug monitoring can be done either proactively to avoid underexposure of drug or reactively when there is perceived loss of benefit, however, more data is required to determine what is the optimum strategy for other biologic agents (53,54,61).
Integrated care of the children with IBD
In addition to disease-specific treatments and associated monitoring, it is important to optimize the macro and micro-nutrition, psychological well-being and bone health. Dietary advice, in general, should focus on a healthy balanced diet composed of fresh foods. It may be important to avoid processed foods high in preservatives and sugars. In patients with intestinal strictures or active colitis, a low fibre diet can be recommended as it may help reduce symptoms. As previously described, EEN is the best-proven treatment option in CD (47,62). The notion that consumption of certain normal dietary factors may perpetuate inflammation has led to multiple dietary intervention studies reporting improvement in symptoms, and in some cases biomarkers, but robust outcomes such as endoscopic healing are lacking. In addition, there are inherent limitations in conducting and interpreting exclusion diet trials, as they cannot be blinded or placebo-controlled (62-66).
Either way in any dietary intervention in pediatric IBD, it is strongly advised that a dietician is involved, to ensure that caloric and nutritional requirements are met. Vitamin and micronutrient deficiencies such as vitamin D, folate, B12 and iron need to be corrected. Adequate levels of vitamin D and recommended daily intake of calcium, minimizing steroid use and good disease control are important in maintaining bone health.
Another important aspect of integrated care that is often forgotten is the emotional well-being of patients with IBD. Anxiety and depression are more common in patients with IBD when compared to the general population. Screening and prompt referrals to a psychologist should be considered at an early stage. Other aspects that affect patients with IBD are feelings of stress, poor self-image, drug-related side effects and dependent behaviours. It is important to equip patients with strategies to deal with some of these such as relaxation and breathing exercises, meditation, light exercise and also building a good support network around them. Finally, to achieve the best outcomes we should invest in a good quality of care that is inclusive, effective and integrated (Figure 3) (67).
Conclusions
The management of pediatric IBD is becoming increasingly complicated as we refine new endpoints such as MH, beyond symptom control. These goals can only be achieved by a prompt diagnosis, in-depth staging and aggressive early treatment of the disease in those with high-risk factors of complications. These measures offer the best chance to potentially alter the natural history of IBD. Newer small molecules targeted therapies are revolutionizing management in IBD, as newer drugs are less immunogenic, safer and are better tolerated.
However, these goals can only be accomplished, when our patients’ values and preferences are incorporated in shared decision making (Figure 3). Lastly, other aspects of preventive health care health promotion should not be ignored to ensure the best outcome for our patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-11/rc
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-11/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-11/coif). ZG has received honoraria for moderating AbbVie sponsored session on how to best use biologics in children with IBD. He does not hold any financial interest or stocks and has not received any other consulting fees in the past 36 months.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Duricova D, Burisch J, Jess T, et al. Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J Crohns Colitis 2014;8:1351-61. [Crossref] [PubMed]
- Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut 2013;62:1505-10. [Crossref] [PubMed]
- Levine A, Sigall Boneh R, Wine E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018;67:1726-38. [Crossref] [PubMed]
- Ng SC, Bernstein CN, Vatn MH, et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013;62:630-49. [Crossref] [PubMed]
- Ng SC, Tang W, Leong RW, et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut 2015;64:1063-71. [Crossref] [PubMed]
- Lui RNS, Ng SC. The same intestinal inflammatory disease despite different genetic risk factors in the East and West? Inflamm Intest Dis 2016;1:78-84. [Crossref] [PubMed]
- Banerjee R, Pal P, Mak JWY, et al. Challenges in the diagnosis and management of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol Hepatol 2020;5:1076-88. [Crossref] [PubMed]
- Ricciuto A, Fish JR, Tomalty DE, et al. Diagnostic delay in Canadian children with inflammatory bowel disease is more common in Crohn's disease and associated with decreased height. Arch Dis Child 2018;103:319-26. [Crossref] [PubMed]
- Schofield JB, Haboubi N. Histopathological Mimics of Inflammatory Bowel Disease. Inflamm Bowel Dis 2020;26:994-1009. [Crossref] [PubMed]
- Moran CJ, Klein C, Muise AM, et al. Very early-onset inflammatory bowel disease: gaining insight through focused discovery. Inflamm Bowel Dis 2015;21:1166-75. [Crossref] [PubMed]
- Mack DR, Langton C, Markowitz J, et al. Laboratory values for children with newly diagnosed inflammatory bowel disease. Pediatrics 2007;119:1113-9. [Crossref] [PubMed]
- van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 2010;341:c3369. [Crossref] [PubMed]
- Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 2006;55:426-31. [Crossref] [PubMed]
- Levine A, Koletzko S, Turner D, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 2014;58:795-806. [Crossref] [PubMed]
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144-64. [Crossref] [PubMed]
- Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn's disease: a population-based cohort study. Gastroenterology 2008;135:1106-13. [Crossref] [PubMed]
- Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135:1114-22. [Crossref] [PubMed]
- Pigneur B, Seksik P, Viola S, et al. Natural history of Crohn's disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis 2010;16:953-61. [Crossref] [PubMed]
- Lee JJ, Escher JC, Shuman MJ, et al. Final adult height of children with inflammatory bowel disease is predicted by parental height and patient minimum height Z-score. Inflamm Bowel Dis 2010;16:1669-77. [Crossref] [PubMed]
- Schoepfer AM, Dehlavi MA, Fournier N, et al. Diagnostic delay in Crohn's disease is associated with a complicated disease course and increased operation rate. Am J Gastroenterol 2013;108:1744-53; quiz 1754. [Crossref] [PubMed]
- Walters TD, Kim MO, Denson LA, et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-α vs an immunomodulator in children with Crohn's disease. Gastroenterology 2014;146:383-91. [Crossref] [PubMed]
- Banerjee R, Pal P, Girish BG, et al. Risk factors for diagnostic delay in Crohn's disease and their impact on long-term complications: how do they differ in a tuberculosis endemic region? Aliment Pharmacol Ther 2018;47:1367-74. [Crossref] [PubMed]
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021;160:1570-83. [Crossref] [PubMed]
- Zubin G, Peter L. Predicting Endoscopic Crohn's Disease Activity Before and After Induction Therapy in Children: A Comprehensive Assessment of PCDAI, CRP, and Fecal Calprotectin. Inflamm Bowel Dis 2015;21:1386-91. [Crossref] [PubMed]
- Carman N, Tomalty D, Church PC, et al. Clinical disease activity and endoscopic severity correlate poorly in children newly diagnosed with Crohn's disease. Gastrointest Endosc 2019;89:364-72. [Crossref] [PubMed]
- Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn's disease: a multicentre inception cohort study. Lancet 2017;389:1710-8. [Crossref] [PubMed]
- Savoye G, Salleron J, Gower-Rousseau C, et al. Clinical predictors at diagnosis of disabling pediatric Crohn's disease. Inflamm Bowel Dis 2012;18:2072-8. [Crossref] [PubMed]
- Kerur B, Machan JT, Shapiro JM, et al. Biologics delay progression of crohn's disease, but not early surgery, in children. Clin Gastroenterol Hepatol 2018;16:1467-73. [Crossref] [PubMed]
- Ziv-Baran T, Hussey S, Sladek M, et al. Response to treatment is more important than disease severity at diagnosis for prediction of early relapse in new-onset paediatric Crohn's disease. Aliment Pharmacol Ther 2018;48:1242-50. [Crossref] [PubMed]
- Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133:423-32. [Crossref] [PubMed]
- Gower-Rousseau C, Dauchet L, Vernier-Massouille G, et al. The natural history of pediatric ulcerative colitis: a population-based cohort study. Am J Gastroenterol 2009;104:2080-8. [Crossref] [PubMed]
- Turner D, Walsh CM, Benchimol EI, et al. Severe paediatric ulcerative colitis: incidence, outcomes and optimal timing for second-line therapy. Gut 2008;57:331-8. [Crossref] [PubMed]
- Turner D, Mack D, Leleiko N, et al. Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology 2010;138:2282-91. [Crossref] [PubMed]
- Schechter A, Griffiths C, Gana JC, et al. Early endoscopic, laboratory and clinical predictors of poor disease course in paediatric ulcerative colitis. Gut 2015;64:580-8. [Crossref] [PubMed]
- Hyams JS, Davis S, Mack DR, et al. Factors associated with early outcomes following standardised therapy in children with ulcerative colitis (PROTECT): a multicentre inception cohort study. Lancet Gastroenterol Hepatol 2017;2:855-68. [Crossref] [PubMed]
- Deva Rajoo G, Tan L, Lopez A, et al. Early Response to Corticosteroid and Baseline C-Reactive Protein Predicts Outcomes in Children with Moderate to Severe Ulcerative Colitis. Dig Dis Sci 2019;64:1929-37. [Crossref] [PubMed]
- Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110:1324-38. [Crossref] [PubMed]
- Grover Z, Burgess C, Muir R, et al. Early mucosal healing with exclusive enteral nutrition is associated with improved outcomes in newly diagnosed children with luminal Crohn's disease. J Crohns Colitis 2016;10:1159-64. [Crossref] [PubMed]
- Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2017;390:2779-89. [Crossref] [PubMed]
- Weinstein-Nakar I, Focht G, Church P, et al. Associations among mucosal and transmural healing and fecal level of calprotectin in children with Crohn's disease. Clin Gastroenterol Hepatol 2018;16:1089-97.e4. [Crossref] [PubMed]
- Theede K, Holck S, Ibsen P, et al. Fecal calprotectin predicts relapse and histological mucosal healing in ulcerative colitis. Inflamm Bowel Dis 2016;22:1042-8. [Crossref] [PubMed]
- Bouguen G, Levesque BG, Pola S, et al. Endoscopic assessment and treating to target increase the likelihood of mucosal healing in patients with Crohn's disease. Clin Gastroenterol Hepatol 2014;12:978-85. [Crossref] [PubMed]
- Bouguen G, Levesque BG, Pola S, et al. Feasibility of endoscopic assessment and treating to target to achieve mucosal healing in ulcerative colitis. Inflamm Bowel Dis 2014;20:231-9. [Crossref] [PubMed]
- Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis 2014;8:1179-207. [Crossref] [PubMed]
- Swaminath A, Feathers A, Ananthakrishnan AN, et al. Systematic review with meta-analysis: enteral nutrition therapy for the induction of remission in paediatric Crohn's disease. Aliment Pharmacol Ther 2017;46:645-56. [Crossref] [PubMed]
- Mack DR, Benchimol EI, Critch J, et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Medical Management of Pediatric Luminal Crohn's Disease. Gastroenterology 2019;157:320-48. [Crossref] [PubMed]
- van Rheenen PF, Aloi M, Assa A, et al. The medical management of paediatric Crohn's disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis 2020; [Crossref]
- Hyams J, Markowitz J, Lerer T, et al. The natural history of corticosteroid therapy for ulcerative colitis in children. Clin Gastroenterol Hepatol 2006;4:1118-23. [Crossref] [PubMed]
- Duricova D, Pedersen N, Lenicek M, et al. The clinical implication of drug dependency in children and adults with inflammatory bowel disease: a review. J Crohns Colitis 2011;5:81-90. [Crossref] [PubMed]
- Pagnini C, Pizarro TT, Cominelli F. Novel pharmacological therapy in inflammatory bowel diseases: beyond anti-tumor necrosis factor. Front Pharmacol 2019;10:671. [Crossref] [PubMed]
- Sabino J, Verstockt B, Vermeire S, et al. New biologics and small molecules in inflammatory bowel disease: an update. Therap Adv Gastroenterol 2019;12:1756284819853208. [Crossref] [PubMed]
- Walker GJ, Harrison JW, Heap GA, et al. Association of genetic variants in NUDT15 with thiopurine-induced myelosuppression in patients with inflammatory bowel disease. JAMA 2019;321:773-85. [Crossref] [PubMed]
- Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019;4:341-53. [Crossref] [PubMed]
- Vermeire S, Dreesen E, Papamichael K, et al. How, when, and for whom should we perform therapeutic drug monitoring? Clin Gastroenterol Hepatol 2020;18:1291-9. [Crossref] [PubMed]
- Dulai PS, Siegel CA, Dubinsky MC. Balancing and communicating the risks and benefits of biologics in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2927-36. [Crossref] [PubMed]
- Hyams JS, Dubinsky MC, Baldassano RN, et al. Infliximab is not associated with increased risk of malignancy or hemophagocytic lymphohistiocytosis in pediatric patients with inflammatory bowel disease. Gastroenterology 2017;152:1901-14.e3. [Crossref] [PubMed]
- Lu Y, Bousvaros A. Immunizations in children with inflammatory bowel disease treated with immunosuppressive therapy. Gastroenterol Hepatol (N Y) 2014;10:355-63.
- Singh AK, Jena A, Kumar-M P, et al. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. United European Gastroenterol J 2021;9:159-76. [Crossref] [PubMed]
- Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut 1993;34:1081-5. [Crossref] [PubMed]
- Hoentjen F, Seinen ML, Hanauer SB, et al. Safety and effectiveness of long-term allopurinol-thiopurine maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis 2013;19:363-9. [Crossref] [PubMed]
- Pouillon L, Ferrante M, Van Assche G, et al. Mucosal healing and long-term outcomes of patients with inflammatory bowel diseases receiving clinic-based vs trough concentration-based dosing of infliximab. Clin Gastroenterol Hepatol 2018;16:1276-83.e1. [Crossref] [PubMed]
- Cohen-Dolev N, Sladek M, Hussey S, et al. Differences in outcomes over time with exclusive enteral nutrition compared with steroids in children with mild to moderate Crohn's disease: results from the GROWTH CD study. J Crohns Colitis 2018;12:306-12. [Crossref] [PubMed]
- Sigall-Boneh R, Pfeffer-Gik T, Segal I, et al. Partial enteral nutrition with a Crohn's disease exclusion diet is effective for induction of remission in children and young adults with Crohn's disease. Inflamm Bowel Dis 2014;20:1353-60. [Crossref] [PubMed]
- Sigall Boneh R, Sarbagili Shabat C, Yanai H, et al. Dietary therapy with the Crohn's disease exclusion diet is a successful strategy for induction of remission in children and adults failing biological therapy. J Crohns Colitis 2017;11:1205-12. [Crossref] [PubMed]
- Svolos V, Hansen R, Nichols B, et al. Treatment of active Crohn's disease with an ordinary food-based diet that replicates exclusive enteral nutrition. Gastroenterology 2019;156:1354-67.e6. [Crossref] [PubMed]
- Levine A, Wine E, Assa A, et al. Crohn's Disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology 2019;157:440-50.e8. [Crossref] [PubMed]
- Lores T, Goess C, Mikocka-Walus A, et al. Integrated psychological care is needed, welcomed and effective in ambulatory inflammatory bowel disease management: evaluation of a new initiative. J Crohns Colitis 2019;13:819-27. [Crossref] [PubMed]
Cite this article as: Grover Z. A narrative review of pitfall and progress in management of inflammatory bowel disease in children. Pediatr Med 2023;6:10.