
Pediatric intracranial ependymoma—a single-center clinical analysis and literature review
Introduction
Ependymoma (EPN) is a glial tumor that originates from the ependymal cells lining the cerebral ventricles and the spinal central canal (1). EPNs can form anywhere in the neural axis, and account for approximately 5% of central nervous system tumors in adults, and 6–10% of central nervous system tumors in children (2). The current standard of care relies on surgical removal and adjuvant radiation therapy, but there is no effective treatment method for recurrent EPN. The concealed locations of tumors make it difficult for clinicians to diagnose the condition early. Once the disease progresses rapidly, it can lead to raised intracranial pressure and herniation, which can be life threatening. This study retrospectively analyzed the clinical data of children with EPN admitted to the Children’s Hospital of Soochow University from October 2011 to February 2021, and compared the results with reports from other studies. We present the following article in accordance with the STROBE reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-83/rc).
Methods
Materials and methods
This study collected and reviewed the clinical data of patients with newly diagnosed pediatric intracranial EPN at the Children’s Hospital of Soochow University from October 2011 to February 2021. In total, 33 patients were enrolled in this study. The retrospective study was approved by the Ethics Board of the Children’s Hospital of Soochow University (No. 2021CS178) and conducted in accordance with the Declaration of Helsinki (as revised in 2013), and the requirement of individual consent for this retrospective analysis was waived.
Before surgery, contrast computed tomography and/or magnetic resonance imaging (MRI) scans of the head were performed on all patients; whole spine MRI scans were performed on patients with no life-threatening conditions to check for metastasis. All patients underwent follow-up examinations. The initial follow-up MRI occurred postoperatively within 72 hours of surgery. The postoperative MRI and the intraoperative findings were used to determine the extent of the surgical resection. The MRI scans were reviewed every 3 months post-surgery for follow-up evaluations.
We investigated Progression-free survival (PFS) and overall survival (OS), and analyzed the prognostic factors. PFS was defined as the interval between the date of surgery and tumor recurrence based on the MRI findings. OS was defined as the interval between the date of initial diagnosis and the date of death.
According to the World Health Organization (WHO) classification system, there are 3 types of EPN: (I) sub-EPN and myxopapillary EPN (Grade I); (II) classic EPN (Grade II); and (III) anaplastic EPN (Grade III). For Grade III patients, adjuvant chemoradiotherapy and postoperative radiotherapy are recommended.
Statistical analysis
SPSS 22.0 software was used for the statistical analysis. The Kaplan-Meier method was used to analyze the survival of all patients, and the log-rank test was used to compare differences between groups.
Results
Clinical characteristics
A total of 33 patients were enrolled in the study. The characteristics of patients are presented in Table 1. Of the patients, 22 were male and 11 were female (the male to female ratio was 2:1). The age of diagnosis ranged from 10 months to 8 years. Of the patients, 14 were aged <3 years old, 10 were aged 3–5 years old, and 9 were aged >5 years old; the median age of diagnosis was 3 years and 5 months. The duration of symptom onsite ranged from 1 day to 1 year, with a median of 15 days. Eleven (32.3%) patients presented with neurological symptoms, including limb movement disorders (5/11), dizziness and headache (3/11), torticollis (1/11), strabismus (1/11), and facial palsy (1/11). The other 22 patients displayed no specific symptoms; however, most of them had gastrointestinal complaints, including vomiting (16/22), changes in appetite (2/22), irritability (1/22), abdominal pain (1/22), and fever (2/22).
Table 1
| Patient characteristics | n [%] |
|---|---|
| Age, years | |
| Median | 3.7 |
| Range | 0.9–11.0 |
| Gender | |
| Male | 22 [67] |
| Female | 11 [33] |
| Tumor location | |
| Supratentorial | 6 [18] |
| Posterior fossa | 27 [82] |
| WHO Grade | |
| WHO primary tumor grade II | 8 [24] |
| WHO primary tumor grade II + III | 5 [15] |
| WHO primary tumor grade III | 20 [61] |
| Clinical manifestations | |
| Limb dyskinesia | 5 [15] |
| Abdominal pain, vomiting | 20 [61] |
| Deviation of the eye and mouth | 3 [9] |
| Dizziness, headache | 3 [9] |
| Fever | 2 [6] |
| Extent of initial resection | |
| Gross total resection | 18 [55] |
| Subtotal resection | 15 [45] |
| Recurrence | |
| Yes | 16 [48] |
| No | 12 [36] |
| Unknown | 5 [15] |
EPN, ependymoma.
Imaging
Of the 33 patients, 6 had supratentorial type cases and 27 had posterior fossa type cases (for further details, Table 1). Two patients had trans tentorial herniation at diagnosis requiring emergency surgery. Seventeen patients received whole spine MRI scans before surgery, but no spinal cord metastasis was found, and only 3 patients showed compression in the medulla oblongata, pons, and abnormal signals in the C1 and C2 areas.
Surgery and pathology
Of the 33 patients, 18 (54.5%) underwent gross total resection, 15 underwent subtotal resection due to tumor location, blood vessel invasion, or brainstem adhesion. In this study, 47 tumor resection operations were performed, including initial diagnosis resections, staged resections, and radical resections after recurrence. A 5-year-old boy died of postoperative respiratory failure due to tumor infiltration into the brain stem, repeated staged operations, and repeated recurrence. This was the only operation-related death in this study.
Pathological and immunohistochemical analyses of the tumors were performed. Eight patients had WHO Grade II tumors, 20 patients had WHO Grade III tumors (including 3 focal anaplasia cases), and 5 patients could not be definitively classified as having either WHO Grade II or III tumors. The clinical parameters are presented in Table 1.
Adjuvant treatment
Five patients received adjuvant radiotherapy and chemotherapy (2 received postoperative radiotherapy for consolidation, and 3 only received adjuvant radiotherapy and chemotherapy after tumor recurrence). Two patients (a 4-year-old and 8-month-old boy and a 5-year-old girl), who both underwent subtotal resection, chose postoperative radiotherapy; 1 survived without progression; 1 recurred after 23 months, and achieved PFS after reoperation. After recurrence, a 6-year-old and 11-month-old boy refused surgery, chose radiotherapy and chemotherapy and achieved PFS; a 5-year-old girl chose surgery combined with radiotherapy and chemotherapy, and achieved PFS; a 5-year-old boy chose surgery combined with chemotherapy, and died of postoperative respiratory failure. Of the 33 cases, 8 cases underwent cerebrospinal fluid diversion surgery due to hydrocephalus.
Follow-up
Of the 33 cases, 5 patients were lost during the follow-up period after surgery, 16 patients had tumor recurrence during the follow-up period, and 12 patients had no recurrence during the follow-up period. Of the 16 recurrence cases, 7 patients were lost during the follow-up period after recurrence had been confirmed, and 9 patients continued to be treated, but 4 of these 9 patients were lost during the follow-up period after second recurrence. The high rate of loss during the follow-up period is a limitation of this study. During the follow-up period, of the 3 patients that showed compression on the whole spine MRI scans at the first visit, 1 patient had tumor recurrence.
Among the 12 patients who had no recurrence during the follow-up period, 6 patients had a follow-up time >36 months, and 3 cases had a follow-up time >5 years (for further details on patients with no tumor recurrence, Table 2). Among those 6 patients with a survival time >36 months, 3 were male, and 3 were female, 1 patient was aged <3 years old, 2 patients were aged between 3 and 5 years old, and 3 patients were aged >5 years old (the patients had a median age of 5 years and 3 months); there were 3 supratentorial type cases, and 3 posterior fossa type cases; 1 case was classified as WHO Grade II, 3 cases were classified as WHO Grade II + III, and 2 cases were classified as WHO Grade III (including 1 focal anaplasia case); 5 patients underwent gross total resections and 1 patient received a subtotal resection but still had residual tumors.
Table 2
| Patient number | Sex/age (years) | Location | Histology (WHO grade) | EOR | Adjuvant therapy | Follow-up period (months)* | Survival status |
|---|---|---|---|---|---|---|---|
| 1 | Male/3.4 | Posterior fossa | III | GTR | – | 2 | Alive |
| 2 | Male/1.9 | Posterior fossa | II | STR | – | 4 | Alive |
| 3 | Male/2.8 | Posterior fossa | III (focal) | STR | – | 3 | Alive |
| 4 | Female/7.2 | Posterior fossa | III | STR | – | 12 | Alive |
| 5 | Male/4.7 | Posterior fossa | III | STR | RT | 13 | Alive |
| 6 | Female/1.1 | Posterior fossa | III | GTR | – | 24 | Alive |
| 7 | Female/2.4 | Supratentorial | II + III | GTR | – | 41 | Alive |
| 8 | Male/4.5 | Posterior fossa | II | STR | – | 48 | Alive |
| 9 | Male/7.8 | Posterior fossa | III (focal) | GTR | – | >60 | Alive |
| 10 | Male/4.5 | Supratentorial | II + III | GTR | – | >60 | Alive |
| 11 | Female/6.0 | Posterior fossa | III | GTR | – | >60 | Alive |
| 12 | Female/6.8 | Supratentorial | II + III | GTR | – | 51 | Alive |
*, followed up until April 1th, 2021. EOR, extent of resection; GTR, gross total resection; STR, subtotal resection; RT, radiotherapy.
Clinical characteristics of recurrent patients
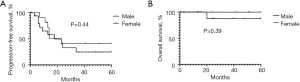
The clinical characteristics of the 16 patients with recurrence were analyzed (for further details on the patients with tumor recurrence, Table 3). Seven (42.4%) patients were aged <3 years old, 4 (30.3%) patients were aged between 3 and 5 years old, and 5 (27.3%) patients were aged >5 years old (the patients had a median age of 3 years). No difference of statistical significance was found when analyzing the relationship between age and tumor recurrence (Figure 1). Ten of the patients were male and 6 were female. No statistically significant difference was found when analyzing the relationship between gender and recurrence (Figure 2A,2B).
Table 3
| Patient number | Sex/age (years) | Location | Histology (WHO grade) |
EOR | Adjuvant therapy | Recurrence- free period (months)# |
Recurrent disease treatment | Follow-up period (months)* | Survival status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female/1.9 | Supratentorial | III | GTR | – | 13 | Surgery | 13 | Loss to follow-up |
| 2 | Female/1.5 | Posterior fossa | II + III | GTR | – | 7 | Surgery | 16 | Loss to follow-up |
| 3 | Male/0.9 | Supratentorial | II | GTR | – | 7 | Surgery | 7 | Loss to follow-up |
| 4 | Male/8.0 | Posterior fossa | II + III | STR | – | 13 | Surgery | 13 | Loss to follow-up |
| 5 | Male/3.0 | Posterior fossa | III | GTR | – | 3 | Surgery | 3 | Loss to follow-up |
| 6 | Male/2.0 | Posterior fossa | II | GTR | – | 24 | Surgery | 24 | Loss to follow-up |
| 7 | Female/1.0 | Posterior fossa | II | STR | – | 14 | Surgery | 14 | Loss to follow-up |
| 8 | Male/2.1 | Posterior fossa | III | GTR | – | 6 | Surgery | 16 | Loss to follow-up |
| 9 | Female/4.2 | Posterior fossa | III | STR | – | 11/3 | Surgery | 15 | Loss to follow-up |
| 10 | Male/3.1 | Posterior fossa | II | STR | – | 23/2 | Surgery | 25 | Loss to follow-up |
| 11 | Male/8.4 | Posterior fossa | II | GTR | – | 6/4 | Surgery | 10 | Loss to follow-up |
| 12 | Male/6.9 | Posterior fossa | III | STR | RT | 34 | Surgery + CT | 40 | Alive |
| 13 | Female/1.7 | Posterior fossa | III | STR | – | 14/3 | Surgery + CT | >60 | Alive |
| 14 | Female/5.0 | Posterior fossa | III (focal) | STR | – | 23/23 | Surgery + RT | >60 | Alive |
| 15 | Male/4.8 | Supratentorial | III | GTR | – | 18/23 | Surgery | >60 | Alive |
| 16 | Male/5.0 | Posterior fossa | III | STR | – | 9/4/5 | Surgery + CT | 20 | Dead |
*, followed up until April 1st, 2021; #, divide multiple recurrence time by /. EOR, extent of resection; GTR, gross total resection; STR, subtotal resection; RT, radiotherapy; CT, chemotherapy.
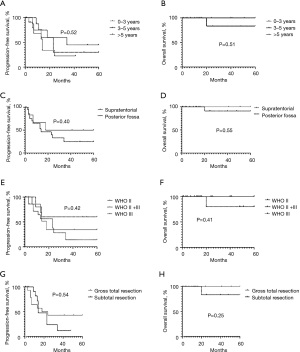
In terms of the location of the tumors, there were 3 (18.8%) supratentorial type cases and 13 (81.2%) posterior fossa type cases. The effect of tumor site on survival was statistically insignificant (Figure 2C,2D). In terms of the WHO classifications, 5 (31.3%) cases were classified as WHO Grade II, 2 (12.5%) cases were classified as WHO Grade II + III, and 9 cases (56.2%), including 1 focal anaplasia case, were classified as WHO Grade III. WHO Grade III patients relapsed more frequently than patients in other groups, but the difference was not statistically significant (Figure 2E,2F).
We also examined effect of the extent of the resection. Eight patients underwent gross total resections, and 8 patients had residual tumors. Patients with residual tumors accounted for 45.5% of all cases, and 50% of all relapse cases. The recurrence rate was slightly higher on patients with subtotal resection than gross total resection without statistic significance (Figure 2G,2H). The main reason for no statistic significance could be the small number of the total patients enrolled in the study, and another possible reason could be that the advancement of the microsurgery technology has reduced the difference between the two surgical resection methods on the recurrence.
The first recurrence time ranged from 3–34 months after surgery (median: 13 months). 15 recurrences happened within 24 months of surgery. Nine of the 16 recurrent patients continued treatment after tumor recurrence. Of these 16 recurrent patients, 8 underwent another surgery, and 1 received palliative care, who to date, has survived with the tumor. Among those 8 patients who underwent second operations, 1 patient was lost during follow-up period after the surgery, and 7 patients had a second tumor recurrence (5 recurred within half a year, and 2 recurred within 2 years). Among the 5 patients who had a second recurrence within 6 months, 3 patients were lost during the follow-up period after the second operation, 1 patient refused radiotherapy but received chemotherapy after the second operation, relapsed again shortly thereafter, and later died of respiratory failure after the fourth operation; and 1 patient survived with palliative tumors. Among the 2 patients who had a second recurrence within 2 years, 1 patient received palliative care, and to date, has survived with the tumor, and the other patient survived and is now cancer-free after undergoing a third-tumor resection and receiving radiotherapy.
Discussion
EPN has the third highest incidence of all pediatric central nervous system malignant tumors (3), and is more common in males than females (4). The male to female ratio in the present study is consistent with the ratios reported in the literature. Forty-two point four percent of patients in the present study were aged <3 years old at onset, and 72.7% were aged <5 years old at onset. The patients had a median age of 3 years and 9 months at onset, which is consistent with data from other domestic pediatric reports (5,6). Research has shown that the younger a patient is aged, the more likely it is that the tumor components will comprise primitive cells, which results in a higher degree of malignancy and worse outcomes (7). However, our findings did not reflect this tendency (Figure 2A,2B).
Unlike adult EPNs, which usually have intracranial and spinal cord involvement, more than 90% of pediatric EPNs only have intracranial involvement, and more than 2/3 of the total cases are located under the tentorium (8,9). No spinal cord EPN cases were found in this study, but 27 (81.8%) of the patients had the posterior fossa type, which supports the conclusion stated above. Experts hold different views on the relationship between tumor location and patient prognosis. Some believe that tumors close to the brainstem and multiple groups of cranial nerves adhere to large blood vessels, nerves, the brainstem, and the medulla oblongata, and there are usually residual tumors after surgeries, which increases the risk of recurrence (6). The prognosis of supratentorial EPN is often poor, due to the late diagnosis, longer course of disease, large tumor size, high pathological grade, and greater chance of metastasis (10). Some researchers have argued that EPNs at different locations may have different molecular mechanisms, and different molecular types lead to different clinical outcomes (11). This study did not confirm whether type (i.e., supratentorial or posterior fossa type) is a prognostic factor (Figure 2C,2D); thus, this issue needs to be explored in future studies.
Surgery is the main method for diagnosing and treating EPNs. The extent of tumor resection and the grade of tumor have been shown to be factors affecting PFS and OS. Maximum safe resection is the priority in EPN treatment. Subtotal tumor resection and WHO Grade III are both predictors of worse outcomes (12,13). Data from Chinese institutions have also verified that the extent of surgical resection and tumor pathological grade are independent predictors of tumor-free survival (14,15). Similarly, in our study, 8 of the 16 recurrent patients underwent subtotal resections, and among the 6 patients followed up with for more than 3 years, only 2 cases did not receive complete resections. This difference was not statistically significant, but this may be due to the limited number of patients in the present study. Only 1 patient died of postoperative respiratory failure. The pediatric EPN operation is difficult, but operation-related mortality is low, and operation is no longer a direct cause affecting prognosis. In recent years, following developments in microsurgery techniques, intraoperative MRI, intraoperative neuro-electrophysiological monitoring and adjuvant radiotherapy, efforts have been made to reduce the effects of residuals on prognosis and recurrence. We did not find any relationship between supratentorial or posterior fossa type and PFS time as has been reported in previous EPN studies; however, this may be due to the small number of patients enrolled in this study (14).
The histopathological grade of EPN is closely related to treatment options and prognosis. The higher the grade, the greater the likelihood of metastasis and relapse (13,16). In this study, 9 (56.3%) patients had WHO Grade III in the recurrence group, and 2 (33.3%) patients had WHO Grade III in the recurrence-free group, which supports this theory; however, in 5 patients with indeterminate WHO grades (Grades II or III), 2 patients experienced relapse, and 3 patients had good outcomes; 2 WHO Grade III focal anaplasia patients had no recurrence, and 1 patient had recurrence, but had a survival time after active treatment >5 years. In this study, the WHO Grade III focal anaplasia cases showed a relatively good healing tendency.
The histopathological system used to grade EPNs has some limitations. Due to tumor heterogeneity, it is difficult to distinguish between Grade II and Grade III cases based only on histopathology results (17). Messahel et al. Reported that the tumor histopathological grade was increased after recurrence, but it is difficult to determine whether this increase was the result of a lack of reliability and repeatability of the histopathological grading system (18). Significant genetic and prognostic heterogeneity have been observed in tumors of the same histopathological grade (19). To address this issue, immunohistochemistry, transcriptome and methylation profiling have been used to assess the risk of EPN in recent years. Pajtler et al. (19) used deoxyribonucleic acid (DNA) methylation profiling to classify ependymal tumors into 9 molecular subgroups and showed superior to histopathological grading for risk stratification. This molecular classification outperforms the current histopathological grading system used in the risk stratification of patients.
Most institutions recognize the role of postoperative radiotherapy. Radiotherapy can reduce local recurrence and improve patient prognosis. The European Association of Neuro-Oncology EPN Task Force Guidelines state that 54 Gy conformal radiotherapy is suitable for children aged 12–18 months, and 59.4 Gy conformal radiotherapy is suitable for children aged >18 months. Due to concerns related to the long-term effects of brainstem necrosis caused by radiotherapy on children’s height, cognition, and endocrine function, many institutions in China, including our center, require children to be aged >3 years old to undergo radiotherapy (6). In our study, only 3 patients were treated with radiotherapy. Two patients, who underwent subtotal resection, received radiotherapy after surgery; among them, 1 survived without progression and 1 had a recurrence after 23 months. Another patient received radiotherapy and chemotherapy after recurrence, and survived without progression. In China, the number of patients who receive radiotherapy is still small, as parents are concerned about the long-term side effects of radiotherapy. With advancements in the precision of radiotherapy and proton therapy, we should be able to have better control over the side effects of radiotherapy, expand the extent of radiotherapy treatment, and reduce the chance of recurrence.
Controversy continues as to the role of chemotherapy. A study suggested that some chemotherapeutic drugs were effective in the treatment of EPN, but the effect on refractory and relapsed EPNs remains uncertain (20). For example, a study suggested that children aged <3 years old who received chemotherapy before receiving radiotherapy had better survival outcomes (6). Currently, the commonly used chemotherapy regimens include cyclophosphamide and vincristine, cisplatin and etoposide, and high-dose methotrexate and temozolomide (21). Three patients in this study received chemotherapy. One patient aged 1 year and 8 months received chemotherapy after the second surgery after recurrence. That patient’s tumor recurred again after 4 months, but the growth of the tumor was very slow, and the patient has survived with the tumor thus far, and has a follow-up time >5 years. One patient aged 5 years old had a tumor that infiltrated the brainstem, and radiotherapy after staged surgical resection was refused. That patient relapsed after 9 months, and underwent chemotherapy after the second operation, but tumor progression could not be controlled. The parents of 1 male patient aged 6 years and 11 months refused to allow their child to undergo surgery after tumor recurrence, chose radiotherapy and chemotherapy, and PFS was achieved. For patients with refractory relapses, the effects of reoperation and radiotherapy or chemotherapy are not ideal. The long-term side effects of chemotherapy are easier for parents to accept than those of radiotherapy, especially among young children. In terms of the choice of chemotherapy timing, it is also recommended to proceed as soon as possible. It is expected that the development of chemotherapy will increase the efficacy of killing residual tumor cells after surgery, and ultimately improve the prognosis of refractory and relapsed EPNs. At present, the International Society of Paediatric Oncology is conducting a phase II trial (NCT02265770) to evaluate the effects of chemotherapy on EPN and the effects of adjuvant chemotherapy on patients who undergo secondary operations. DNA methylation and histone deacetylation inhibitors also represent new research directions (22).
The recurrence rate of pediatric EPN is very high, and with a 5-year survival rate of only 25%, the prognosis of recurrent EPN is poor (23). In this study, the first recurrence rate was 57.1%, and first recurrence mostly occurred within 24 months of surgery. After recurrence, 8 patients underwent a radical second operation, but 7 patients relapsed again, and 1 patient died of postoperative complications. The second recurrence showed 2 peaks within 6 months and around 24 months after the second operation. After recurrence, the recurrence rate for patients who undergo aggressive surgery and adjuvant treatment is still high (23). It is believed that even continued active treatments cannot completely remove tumors, but such treatments can delay the growth of tumors and prolong the life of patients. We also observed this phenomenon in our study where the survival time of 3 patients who were followed up with after the radical second operation was >60 months.
This study had a number of limitations. First, the lack of statistically significant results is unsatisfactory. DNA methylation, gene detection, and other new technologies were not widely used in this clinical setting. The rate of patient lost during the follow-up period was high given the high recurrence of EPN. However, due to a lack of confidence in treatments, parents tend to abandon treatment or seek treatment at multiple institutions.
For pediatric EPNs, future research should seek to continuously improve the accuracy of surgery, further refine and improve tumor risk classification, increase the proportion of patients who receive postoperative radiotherapy, and improve the chemotherapy regimens. It is hoped that joint efforts may reduce the chance of relapse and improve the prognosis of patients with refractory or relapsed EPNs.
Acknowledgments
The authors would like to thank Ms. Chenchen Sun for her translation of the manuscript from Chinese to English.
Funding: This study was supported by NSFC (No. 81802499), the Jiangsu Project (Nos. CXTDA2017014 and BE2019672), the Suzhou Project (Nos. SS201809, GSWS2020039, and KJXW2018016), and the National Clinical Research Center for Hematological Disorders (No. 2020ZKPB02).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ibrahim Qaddoumi, Anthony Liu and Chenchen Sun) for the series “Pediatric CNS Tumors in China” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-83/rc
Data Sharing Statement: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-83/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-83/coif).The series “Pediatric CNS Tumors in China” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Board of the Children’s Hospital of Soochow University (No. 2021CS178), and the requirement of individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stenzel AE, Fenstermaker RA, Wiltsie LM, et al. Disparities among racial/ethnic groups of patients diagnosed with ependymoma: analyses from the Surveillance, Epidemiology and End Results (SEER) registry. J Neurooncol 2019;144:43-51. [Crossref] [PubMed]
- Ostrom QT, de Blank PM, Kruchko C, et al. Alex’s lemonade stand foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol 2015;16:x1-x36. [Crossref] [PubMed]
- Kilday JP, Rahman R, Dyer S, et al. Pediatric ependymoma: biological perspectives. Mol Cancer Res 2009;7:765-86. [Crossref] [PubMed]
- McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: a surveillance, epidemiology, and end results study. J Neurosurg 2009;110:725-9. [Crossref] [PubMed]
- Amirian ES, Armstrong TS, Aldape KD, et al. Predictors of survival among pediatric and adult ependymoma cases: a study using Surveillance, Epidemiology, and End Results data from 1973 to 2007. Neuroepidemiology 2012;39:116-24. [Crossref] [PubMed]
- Han Z, Li M, Liu W, et al. Therapeutic effects of intracranial ependymoma in children. Chin J Neurosurg 2019;35:765-70.
- Sun Y, Zhang Y. Treatment progress and prognosis of ependymoma in children. Chin J Neurosurg 2004;20:266-8.
- Wu Liu P, Zhao F. Advances in molecular biology of ependymoma. Chin J Neurosurg 2019;35:207-9.
- Gerstner ER, Pajtler KW. Ependymoma. Semin Neurol 2018;38:104-11. [Crossref] [PubMed]
- Sayegh ET, Aranda D, Kim JM, et al. Prognosis by tumor location in adults with intracranial ependymomas. J Clin Neurosci 2014;21:2096-101. [Crossref] [PubMed]
- Lukashova-v Zangen I, Kneitz S, Monoranu CM, et al. Ependymoma gene expression profiles associated with histological subtype, proliferation, and patient survival. Acta Neuropathol 2007;113:325-37. [Crossref] [PubMed]
- Leeper H, Felicella MM, Walbert T. Recent advances in the classification and treatment of ependymomas. Curr Treat Options Oncol 2017;18:55. [Crossref] [PubMed]
- Nuño M, Yu JJ, Varshneya K, et al. Treatment and survival of supratentorial and posterior fossa ependymomas in adults. J Clin Neurosci 2016;28:24-30. [Crossref] [PubMed]
- Li Chen, Yao Y, Wang Y, et al. Clinical features and survival analysis of 93 cases of ependymomas. Chin J Neurosurg 2013;29:1087-9.
- Bian Y, Chen W, Li Z, et al. Analysis of survival and prognostic factors of patients with intracranial ependymoma. Chin J Clin Oncol 2019;46:138-44.
- Hollon T, Nguyen V, Smith BW, et al. Supratentorial hemispheric ependymomas: an analysis of 109 adults for survival and prognostic factors. J Neurosurg 2016;125:410-8. [Crossref] [PubMed]
- Ellison DW, Kocak M, Figarella-Branger D, et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed 2011;10:7. [Crossref] [PubMed]
- Messahel B, Ashley S, Saran F, et al. Relapsed intracranial ependymoma in children in the UK: patterns of relapse, survival and therapeutic outcome. Eur J Cancer 2009;45:1815-23. [Crossref] [PubMed]
- Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell 2015;27:728-43. [Crossref] [PubMed]
- Khatua S, Ramaswamy V, Bouffet E. Current therapy and the evolving molecular landscape of paediatric ependymoma. Eur J Cancer 2017;70:34-41. [Crossref] [PubMed]
- Delgado-López PD, Corrales-García EM, Alonso-García E, et al. Central nervous system ependymoma: clinical implications of the new molecular classification, treatment guidelines and controversial issues. Clin Transl Oncol 2019;21:1450-63. [Crossref] [PubMed]
- Shen L, Kantarjian H, Guo Y, et al. DNA methylation predicts survival and response to therapy in patients with myelodysplastic syndromes. J Clin Oncol 2010;28:605-13. [Crossref] [PubMed]
- Qi H, Guo H, Zeng W. Progress in clinical research on childhood ependymoma. J Int Neurol Neurosurg 2020;47:667-72.
(English Language Editor: L. Huleatt)
Cite this article as: Lu Y, Li B, Li Z, Lu J, Du W, Lu Q, Meng L, Wang H, Hu S. Pediatric intracranial ependymoma—a single-center clinical analysis and literature review. Pediatr Med 2022;5:23.

