
Primary cilia in the development of the cerebral cortex: a literature review
Introduction
Primary cilium (also called non-motile cilium) is mainly composed of a basal body, a transition zone, an axoneme, and a ciliary membrane (1). Primary cilia present in the brain where they play a part in postnatal cortical development and homeostasis (2). Interestingly, they remained mysterious and controversial until the end of the 1960s (3). Some researchers regarded them as useless cell structures, while others thought that they violated the general principles of cell economy (4). The structure and function of primary cilia were not intensively studied until technical advances were made in high-resolution electron microscopy. Generally, there is only one unique cilium per cell, and the abnormality of structure and function of this cilium may cause Bardet-Biedl syndrome (BBS), Oral-facial-digital syndrome type I (OFD1), Meckel Gruber syndrome (MKS), and other genetic diseases involving multiple organs (5,6). Due to overlapping cilium-related clinical phenotypes associated with these diseases, they are also called ciliopathies (6). Duncan (7) used a Transmission Electron Microscope (TEM) to make the first report of primary cilia on cells of the neural tube. He found, in chickens, a single cilium on the luminal surface of each neural tube cell. Although neural cilia are similar to other primary cilia in ultrastructure, how they affect the developing cerebral cortex remains unknown (8).
Objectives
Based on previous reports, we aim to systematically highlight the critical roles of primary cilia in the proliferation and differentiation of neural progenitor cells, and the migration of newborn neurons, as well as the transduction of signaling. Another aim of this review is to provide guidance for theorizing about the pathogenesis and treatment of cilia-related cortical diseases. We present the following article in accordance with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-107/rc).
Methods
We performed the literature search using online database PubMed from 1957 to 2021. The search terms included “cortical development”, “developing cortex”, “primary cilia”, “primary cilium”, and “ciliopathies” (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | August 30, 2021 |
| Databases and other sources searched | PubMed |
| Search terms used | cortical development, developing cortex, primary cilia, primary cilium, ciliopathies |
| Timeframe | Literature published from January 1957 to August 2021 |
| Inclusion criteria | Study type: Review, Systematic Review, Case report, Books and Documents Language restrictions: English |
| Selection process | One author conducted the selection. The other authors reviewed the intended citation and added suggestions. Agreement was reached after discussion. |
Discussion
Ciliary systems
Microstructure of primary cilia
As is shown in Figure 1 (right panel), the structure of a primary cilium includes, from bottom to top, a basal body, a transition zone (TZ), an axoneme, and a ciliary membrane.
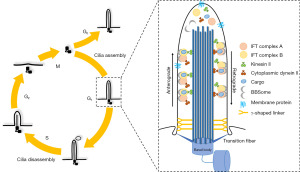
The basal body forms the base of the cilium, and the basal foot and transition fibers are adjacent to the TZ at the distal end of the basal body (9). The TZ is a “zone” around the bottom of the axoneme that is just above the basal body. Y-shaped fibers connect the axonemal microtubules inward and the ciliary necklace membranous particles outward. TZ and transition fibers of the basal body act as a “ciliary gate” to control cytosolic components and ciliary membrane entry to and exit from cilia (10-12). The axoneme, attached to the basal body, is the skeletal structure of a cilium and is composed of microtubules and their accessory proteins. The ciliary membrane is a lipid bilayer derived from Golgi-associated vesicles and connects with cell membrane (13). The primary cilia act as specialized signaling hubs to integrate diverse developmental and homeostatic information, and dynamically regulate downstream effectors.
Although some early cross-section Electron Microscope (EM) studies have challenged the classically ‘9+0’ pattern of primary ciliary axonemal microtubules, its ultrastructure was unknown until recently (14,15). The application of serial electron tomography and cryo-electron microscopy, the combined cryo peel-off method, provided the unprecedented insights. Researchers found that the microtubule complexes of a primary cilium in a kidney’s epithelial cells terminate at different locations and that ciliary diameters reduce toward the ciliary tip, which is consistent with the reversible bending property of a ciliary axoneme (16,17).
Intraflagellar Transport (IFT)
The maintenance of a cilium must rely on the bidirectional transport system (also called the IFT) between the ciliary tip and the cell body for they cannot synthesize proteins by themselves (18). IFT is mainly formed from IFT protein complexes (IFT A and IFT B), and motor proteins (Kinesin II and cytoplasmic dynein II), which together constitute the “power train” for material transportation in the cilium (19). Interestingly, single-particle tracking localization microscopy has found that IFT proteins move at different rates in different regions of the cilium (20). IFT proteins move slowly in the distal appendages (DAPs) and TZ, and much faster in the proximal TZ and ciliary compartment (CC).
Overall, there are two types of IFT: anterograde transport and retrograde transport. Anterograde transport is driven by kinesin II, in which one end interacts with axoneme microtubules, the other anchors on the IFT B, carrying “ciliary cargo” and IFT A, from the ciliary base to the ciliary tip (21). After arriving at the ciliary tip, the “ciliary cargo” is unloaded, and at the same time, the IFT protein complexes are reshaped, so that retrograde transport can start (22). Driven by cytoplasmic dynein II, one end joins to the axoneme microtubules, the other interacts with IFT A, carrying useless substances and IFT B from the ciliary tip back to the cell body for degradation or reuse (23,24).
Assembly and disassembly of primary cilia
The assembly and disassembly of primary cilia are regulated by the cell cycle, depending on IFT to furnish the transport and exchange of materials (Figure 1, left panel). The mother centriole becomes the basal body which anchors on the ciliary membrane through transition fibers and the basal foot in the G0/G1phase. When IFT participates in the transportation of cilia-related proteins, new primary cilia initiate in form, and further elongate under the control of multiple signaling.
When cells re-enter into the cell cycle upon stimulation with serum, the depolymerization of primary cilia is activated. Pugacheva et al. (25) find that after stimulating serum-starved cells for 1–2 hours, HEF1 (pro-metastatic scaffolding protein)-Aurora A (a centrosomal kinase) is induced to activate and promote cells’ entry into the M phase. Meanwhile, it also stimulates HDAC6-dependent tubulin deacetylation through the cascade phosphorylation of HDAC6 (a tubulin deacetylase), destroying ciliary stability. Later, other researchers discover that CEP (centrosomal protein)55 can interact with Aurora A to regulate the stability of Aurora A and promote cilia disassembly (26,27). In addition, cilia disassembly also requires the participation of actin dynamics. The latest findings have shown that F-actin can accumulate in primary cilia to remove cilia tips for cilia decapitation, triggering cilia disassembly (28). Other researchers, using a cell/cilia cycle biosensor for single-cell kinetics, discover that actin-mediated ciliary scissions are beneficial all along the ciliary cycle, and can also contribute to ciliary growth (29). Furthermore, the common thought that cilia initiate disassembly from G1 to S has been contested. The same researchers observe that cilia can transit from G1/S to S/G2/M-phase in NIH/3T3 cells. The relationship between cilia and the cell cycle is more complex than we used to think, and thoroughly studies are needed.
Despite how trivial they may look, the integrity and stability of the primary cilia perform vital functions in regulating various biological processes in living systems. Primary cilia anomalies will generate ciliopathies, and the pathogenesis of these illnesses awaits further investigation.
Ciliopathies
In recent years, ciliopathies, which affect multiple organ systems and tissues, have been defined as a group of diseases resulting from dysfunctions of cilia (30). Genetic and phenotypic heterogeneity and overlaps make it sometimes difficult to determine the classification of these diseases, which inhibits clinical diagnosis and treatment. Common diseases include BBS, JBTS (Joubert syndrome), OFD1, and MKS, involving the brain, kidney, and heart, etc. Here we will focus on BBS, OFD1, and MKS with severe brain phenotypes and summarize the brain morphological defects (see Table 2).
Table 2
| Ciliopathy | Mutated genes | Morphological defects | References |
|---|---|---|---|
| BBS | BBS1-19 | Reduced total gray matter volume; cortical enlargement in the occipital lobe; hippocampal dysgenesis; cerebellar atrophy | (31-35) |
| OFD1 | Ofd1, C2CD3, INTU, KIAA0753, IFT57, C5orf42, TMEM107/138/216/231, WDPCP, TCTN3, DDX59, NEK1, TBC1D32, SCLT1 | Agenesis of corpus callosum and cerebellar vermis, congenital cerebral cysts, porencephaly | (36-39) |
| MKS | Mks1, TMEM67/138/216/231/237, CEP90, RPGRIP1L, CC2D2A, NPHP3, TCTN2, B9D1/2, EVC2, C5orf42, SEC8 | Occipital meningoencephalocele | (40-43) |
| JBTS | Arl13b, Inpp5e, NPHP1, AHI1, CEP290, RPGRIP1L, TEME67, CC2D2A | Axonal tract malformation, cerebellar ataxia | (44,45) |
The central nervous system related symptoms of BBS include cognitive impairment, ataxia and hearing loss, etc. (35). Currently, the proteins encoded by 19 genes (BBS1-BBS19) have been uncovered to be involved in lipid homeostasis, IFT, establishment of cell polarity, and regulation of centrosome functions. The mouse model of BBS shows increased apoptosis, decreased neurogenesis, and then progression into neonatal hydrocephalus (46). In vitro, BBS mutations cause impaired neurite outgrowth and longer cilia (47).
The common neurological symptoms of OFD1 consist of brain structural anomalies, mental retardation, and cerebellar hypoplasia etc. The OFD1 protein is of great importance to the formation of primary cilia (48). Findings of in vitro studies which show no primary cilia and abnormal Shh and Wnt signaling pathways of Ofd1 mutants are in line with lack of ciliary axoneme and defective Dorso-Ventral patterning in vivo (49,50).
MKS is the most severe and lethal type of ciliopathy, and is characterized by occipital encephalocele (51). Double mutant of Mks1 (encoding TZ protein) and BBS4 in mouse models exhibit remarkable defects in the structure of cilia and signaling pathways than either single mutant, indicating that not only the phenotypes of distinct ciliopathies overlap, but multiple mutations contribute to severe outcomes (52).
Briefly, brain specific phenotypes presented by the primary cilia in biological process emphasis its vital function. Next, we try to look back to the researches on the primary cilia in the developing cortex to figure out how it works in it.
The roles of primary cilia in cortical development
The cerebral cortex is a highly organized structure that contains about 86.06 billion neurons (53). Primary cilia occupy roughly 3.2×109 µm2 space, presenting on progenitors neurons and differentiated neurons (54,55). Cilia genes follow rhythmic circadian patterns of expression in the brain (56). The high dynamics of the structural and functional components of cilia drive metabolic, physiological, and behavioral processes of developing cortex (57). In this part, we will introduce the roles of primary cilia in the proliferation and differentiation of neurons, the migration and synaptic growth of neurons, and how they mediate signaling pathways. We summarize the genes/proteins associated with ciliogenesis discussed there (see Table 3).
Table 3
| Gene/protein | Localization | Functions | Impact on cortical development |
|---|---|---|---|
| Arl13b | Cilia | Cilia assembly, protein trafficking and Shh signaling (58) | Polarized radial glial scaffold formation, migration and placement of interneurons (59,60) |
| BBS 1/4/5/7/9/10/11/12 | Cilia | BBSome assembly and cilia protein trafficking (61) | Thinning of the cerebral cortex (34) |
| CEP170 | Basal body | Spindle assembly and cilia disassembly (62,63) | Microcephaly (63) |
| CPAP | Basal body | Centriole biogenesis and cilia disassembly (64) | Slower neuronal migration, aberrant neuronal morphology, microcephaly, increased axonal length (65-67) |
| Dync2h1 | Cilia/Basal body | Cilia protein trafficking (68) | Loss of Shh in the neural tube (68) |
| Fbxo41 | Basal body | Cilia disassembly and Shh signaling (69) | Regulates neuronal cilia structure and signaling capacity (69) |
| Ftm (Rpgrip1l) | TZ | TZ assembly (70) | Shortened neurogenic period, increased newborn Ips (71) |
| Gpr161 | Ciliary membrane | Antagonize Hh signaling (72) | Increased IPs and basal RG, thinner cortex (73) |
| IFT27 | Cilia/Basal body | Cilia protein trafficking and Shh signaling (74) | Loss of Shh in the neural tube (74) |
| IFT88 | Ciliary axonemes/Basal body | Cilia protein trafficking (75) | Subpial heterotopias in the forebrain, microcephaly (75,76) |
| IFT172 | Cilia/Basal body | Cilia protein trafficking (77) | Perturb NPC proliferation and neuronal migration (77) |
| Inpp5e | Ciliary membrane | Regulate ciliary stability (78) | Increased neuronal formation, cortical malformations (79) |
| Katanin p80 | Basal body | Ciliogenesis and Shh signaling (80) | Microcephaly with simplification of cortical gyri and sulci (80) |
| Kif2a | Cilia/Basal body | Cilia disassembly (63) | Microcephaly, cortical malformations, neuronal migration (81,82) |
| Kif3a | Cilia/Basal body | Cilia assembly (83) | Delay neuronal migration and differentiation (83) |
| TMEM67 | TZ | Cilia protein trafficking and signaling (84) | Hydrocephalus, neural tube defects (84,85) |
| TMEM216 | Basal body | Ciliogenesis and centrosomal docking (86) | Anomalies of occipital cortex (87) |
| Tulp3 | Cilia | Ciliary protein trafficking, antagonize Hh signaling (88) | RGs malformation (89) |
| WDR62 | Basal body | Centriole biogenesis and cilia disassembly (63,75) | Microcephaly, loss and premature differentiation of RGs (63,75) |
TZ, transition zone; IPs, intermediate progenitors; RG, radial glia; NPC, neural precursor cell.
Participation in the proliferation and differentiation of neural cells
Primary cilia control symmetric and asymmetric divisions of NPCs through cell cycle dynamics (90). In early stages, NPCs divide symmetrically to expand the neural stem cell pool (Figure 2A). In neurogenesis, most of the radial glial cells (RGCs) divide asymmetrically to generate neurons in a different way (91,92). At this stage, the cilia of the future neurons regrow on the lateral cell membrane instead of on the apical one (Figure 2B) (93). Primary cilia, exist in the G0/G1 phase, are gradually absorbed when cells re-enter into the cell cycle (S phase) (94). Surprisingly, part of the ciliary membrane is conserved during asymmetric divisions and remains attached to the mother centriole. The daughter cell that inherits this mother centriole has increased probability to remain a progenitor (95).
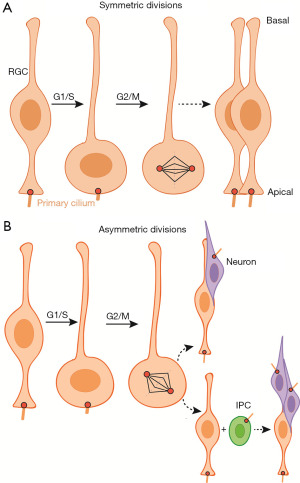
Aberrant CPAP (a centrosomal-P4.1-associated protein) that leads to primary microcephaly is the most typical example (65). CPAP is a negative regulator of ciliary length in dependent of its role in centrosome biogenesis (64,96). Its mutation will cause abnormal cilia disassembly, prolong the G1/S phase, and consequently, reduce the proliferation of NPCs and increase apoptosis. Slender primary cilia are also found in brain organoids derived from microcephaly patients with CPAP mutation, which is in keep with other, results acquired in 2-D culture system. Further studies have discovered that deletion of murine CPAP produces formation of monopolar spindles in radial glial progenitors (RGs) and secondary severe apoptosis (97). WDR62, regulates cilia disassembly when interacting with CEP170 and Kif2a, and cilia formation when interplaying with CPAP/IFT88, is the second common mutated gene correlated with microcephaly (63,75,98). Both knock out (KO) mouse models and human cerebral organoids reveal that WDR62 mutants create decreased proliferation and premature differentiation of NPCs. Interestingly, ciliogenesis and neurogenesis defects are more robust in cerebral organoids than in mutant mice (63). This is because unique outer radial glia cells (oRGs), promoting massive expansion of neural stem cells, abundantly occur in human cerebral cortex with rare presence in rodent cortex. Thus, mouse models failed to recapitulate some human disease biology seen in human patients. In contrast, brain organoids provide an advantage as they can uncover molecular mechanism of developing brain in incomparable detail (99).
In other research, IFT88, a component of IFT B complex, inducing ciliary formation. Kif3a, a member of the kinesin II family required for cilia protein trafficking and growth. Conditional depletion of IFT88 or Kif3a at both early (E10.5) and late (E13.5) stages of mice resulted in little impact on progenitor proliferation, except in a small region where cilia-dependent Hh signaling is significant to its proliferation (100). In addition, different phenotypes were discovered in mice with different gene mutations. Ftm, located at the ciliary TZ, is necessary for the TZ localization of many other ciliopathy proteins (101). Inpp5e, a phosphoinositide 5’-phosphatase that hydrolyzes PIP2 and PIP3, stabilizes cilia structure and length (102). In Ftm mutants, the initiation of cortical neurogenesis is delayed, which can be compensated for in later stages (71). While in Inpp5e mutants, the defects of neurogenesis can be rescued by restoring Gli3 repressor (79). The impairment of mutant mice (Ift88, Kif3a and Ftm) is due to a disorder of ventro-dorsal polarization which can be easily explained by the role of signal transduction played by the cilia. We will discuss in detail later. Together, these data suggest complex roles of cilia in corticogenesis, which deserve further studies.
Regulating newborn neuronal migration and growth of dendrites/axons
Neuronal migration is a precisely mediated process, which takes neurons from their location of origin to their destination in the cortex. Aberrant migration of neurons can alter the formation of neuronal circuitry and result in severe functional defects, such as epilepsy and mental retardation (103,104).
The apico-basal polarized RGCs act as a “scaffold” for cell migration in the developing cortex, coordinating correct radial migration of neurons from the ventricular zone (VZ) to the cortical plate. In the experiment where 30 cilia-related genes were knocked down, knockdown of BBS1, BBS7, BBS10, and TMEM216 changed the apico-basal polarity of RGCs (105). In vivo, the regulation of IFT172 (a component of the IFT complex) in the germinal zone of the embryonic mouse brain also disrupts the radial migration of neurons (77). Beyond that, Arl13b, a GTPase enriched in cilia, is responsible for the initial formation of the polarized RGCs scaffold. Arl13bhnn/hnn mutants show short cilia and the polarity reversal of RGCs, subsequently, the neurons generated from NPCs migrate abnormally near the cortical surface, and eventually neuronal layered structure breakdown (59).
Unlike excitatory glutamatergic neurons (ENs), inhibitory GABAergic interneurons (INs) migrate long distances from the medial ganglionic eminence (MGE) to reach the cerebral cortex (106-108). Migrating interneurons can assemble primary cilia to maintain proper interneuron trajectory and balance excitatory and inhibitory activity of nervous system (109,110). Hnn mutation and the abnormality of Kif3a, IFT88 lead to incorrect tangential migration, such as process branching, travel distance shortening, and even failure to leave their tangential migrating streams efficiently (60,110). In the developing cortex, MGE cells and cells in the migrating pathway express N-cadherin, which can maintain cell polarity over long distance migration (111). Interestingly, MGE cells that migrate on N-cadherin substrates, rather than on laminin, exhibit fast synchronous centrosomal and nuclear movements, and reduced ciliogenesis (112). Therefore, N-cadherin influences both the cell polarity of migrating MGE cells and centrosomal movements and ciliogenesis.
The appropriate growth of dendrites and axons is necessary to synapse formation and connections, and is also essential for the accurate and specific functioning of the nervous system (113). In a mouse model, conditional cilia deletion of adult-born hippocampal neurons induced disruption in dendritic and synaptic integration, and enhanced Wnt/β-catenin signaling was required for dendritic refinement (114). Later evidence has shown that cilia also regulated the growth of the dendrites of projection neurons in developing neocortical neurons. Overexpression of ciliary 5-HT6 or Kif3a impairs normal ciliogenesis and dendrite outgrowth, which can be rescued by coexpression of type III adenylyl cyclase (ACIII, proteins enriched in neuronal cilia) with 5-HT6 (115). Intriguingly, in another study, primary cilia activated cilia-localized insulin-like growth factor1 receptor (IGF-1R) and downstream Akt signaling to protect dendrites of immature neurons from alcohol and ketamine (116). Deletion of Arl13b and Inpp5e led to altered axon growth behavior, such as misoriented axonal tracts and reduced formation of branching protrusions (45). This was explained by the deletion of Arl13b, which deregulated ciliary-PI3K/AKT. On the contrary, Cenpj silencing, exhibits enhanced microtubule stabilization, more branches and larger growth cone area, might be a novel target for axonal regeneration (67).
Mediating signaling pathways
Primary cilia maintain multiple cortical developmental processes such as neural tube patterning, and neural cell proliferation, as well as neural cell division for its membrane has dense lipid rafts to convey a wide range of signals, such as the signaling pathways of sonic hedgehog (Shh), Wnt, and the mTOR (117-119).
Shh is a member of the Hh family, and is extensively expressed in the central nervous system. The overall patterns are summarized in Figure 3 (120). The activation of Shh signaling pathway depends on the presence or absence of Shh ligands, producing Gli transcription factors (GliAs) or Gli repressor forms (GliRs), respectively (10). Smo’s ciliary level is regulated by the ubiquitination state of the receptor. IFT27, a component of IFT B complex, is required for BBSome trafficking matters for Hh signaling (121). BBSome controls the assembly and recycling of cilia-related proteins from ciliary base to tip. Blocking ubiquitination of Smo by disrupting IFT27 and BBSome, Smo accumulates in the cilia without pathway activation (122). Smo is a critical Shh pathway component, for which abnormality can cause severe developmental disorders (123). Tulp3, an adaptor protein, regulates the trafficking of the Arl13b into cilia (88). Without Shh ligands, the primary cilia-localized orphan Gpr161, a G-protein-coupled receptor (GPCR) controlled by Tulp3/IFT-A, represses the activation of the Shh pathway. Gpr161 increases cAMP levels in a GαS-coupled manner, and combines protein kinase A (PKA, cAMP-activated kinase) with the Shh signaling pathway (88,124). Deletion of Gpr161 in mid-gestation of a mouse causes increased Shh signaling, further leading to hydrocephalus, ventriculomegaly, and periventricular nodular heterotopia (73).
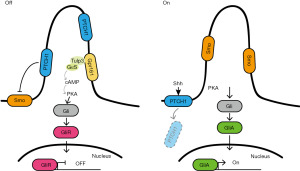
Unusual primary cilia lead to a defective ventral neural tube, which is patterned by a gradient of Shh secreted from the notochord and floor plate (125). Mutations in genes involved in trafficking of molecules within the cilia give rise to different impacts on the neural tube along the anterior-posterior axis. Dync2h1, a subunit of dynein II, drives ciliary retrograde IFT (126). A mutation in Tulp3 results in an up-regulation of Shh signaling in the posterior dorsal domain, while a mutation in Dync2h1 results in down-regulation of Shh in the anterior regions of the spinal cord (127). Besides an impaired neural tube, abnormal neuron migration or location, caused by cilia-dependent Shh destruction, also leads to developmental abnormalities such as microcephaly, and craniofacial malformation (59). Meanwhile, Fbxo41 and katanin p80 negatively regulate the length of cilia. Both cilia disassembly provoked by the accumulation of Fbxo41, and excessive ciliogenesis with the loss of katanin p80, have an effect on the Shh signaling transduction capability (69,80).
The Wnt signaling pathway is another key regulatory pathway in cortical development (128). But the relationship between primary cilia and Wnt signaling transduction (mediate, suppress or irrelevant) is still controversial at present (129). Some studies have reported that defective primary cilia do not affect Wnt/β-catenin signaling in zebrafish and mice (130,131). Others demonstrated that the cerebella of TMEM67 mutant mice were hypoplastic and showed up-regulation of the β-catenin-dependent canonical Wnt pathway, increased proliferation, and apoptosis (84). In contrast, there is general agreed that the non-canonical Wnt [planar cell polarity (PCP)] signaling pathway can target thin-layer cells, which regulate cell aggregation and elongation, so as to close the neural tube (132).
Furthermore, primary cilia regulate ventricle morphogenesis and corticogenesis, via modulation of the mTOR pathway. Mutations in MTOR contribute to reduced neuronal cilia in patients with focal malformation of cortical development (FMCD) by disturbing Wnt signaling (133). Cilium mutants result in disinhibition of mTORC1, impaired mitotic spindle orientation, increased RGCs, enlarged ventricles, gradually form hydrocephalus (134). In contrast, overactivation of the mTORC1 caused by the loss of STRADA, a pseudokinase and an upstream regulator of mTORC1, displays disrupted primary cilia and megalencephaly (135,136). The relationship between primary cilia and mTOR pathway is a complex one. It is likely that these processes are mutually influential.
Taken together, the sophisticated signaling pathways, which play critical roles in neurogenesis, are regulated by primary cilia, and we have touched on just the tip of the iceberg in this review. How these pathways intertwine with cilia-related genes to maintain correct neurogenesis needs to be explored in greater detail.
Limitations
The wide range of sub-topics covered in this review may lead to the discussion of each sub-topics not deep enough. The tables of the summaries of associated brain morphological defects and cilia-related genes/proteins are not comprehensive enough.
Outlook and conclusions
With the development of molecular biology, bioinformatics, and other technologies in recent years, researchers now have a deeper knowledge of the ciliary systems and their roles in the growth and development of organisms and the maintenance of homeostasis. The role of primary cilia in cerebral cortical development has become increasingly clear from published studies. Firstly, primary cilia not only affect the proliferation and differentiation of NPCs by regulating the cell cycle, but also affect neuronal migration and the growth of dendrites/axons. Secondly, primary cilia act as the “signaling enhancement receiver” of cells. Numerous receptors, specific to different signaling pathways, are located on a primary cilium’s membrane, which receives and integrates various signals in the environment, and regulates the downstream effectors. Abnormalities of primary cilia will directly or indirectly affect the normal development of the cerebral cortex and cause different brain deformity and dysfunction. These clinically overlapping disorders, also known as ciliopathies, need to be revealed by future deep investigations.
Although we have a new understanding about the link between ciliogenesis and neurogenesis, many issues still remain to be investigated with respect to primary cilia and cortex development:
- The role of primary cilia in the Shh signaling pathway has been studied thoroughly, but whether the canonical Wnt signaling pathway requires the participation of primary cilia in cortical development is still unclear. What other signaling transduction pathways are there? How do they form a large signaling pathway network?
- Many cilia-related genes have been discovered so far. How do they interact with each other to achieve the precise regulation of cortical development in a timely and well-spaced manner? What are the upstream and downstream relationships of these genes?
- Neurons communicate with each other through dendrites and axons. There are several papers addressing this topic, but more work needs to be done to explore deeper mechanisms. And what neurological diseases are involved? Can we find a therapeutic target?
In contrast with excitatory and inhibitory neurons, researches on astrocytic and oligodendrocytic primary cilia lags far behind (137,138). Further studies of these questions are needed.
Assembly and disassembly of primary cilia are tightly coupled to the cell cycle. How do the structure of primary cilia change when neurons become mature and stay in G0 phase for a long time? What proteins are involved in regulation?
In the future, we can use two approaches to seek answers to these questions (54). One is the observation of animal models and human brain tissues, using IUE techniques or advanced brain imaging techniques (139). Another essential and powerful approach will be the study of brain organoids, the 3-D culture system obtained from human embryonic stem cells (hESCs), or patient-derived induced pluripotent stem cells (iPSCs) in vitro (140). With advances in CRISPR/Cas9 and single cell sequencing, brain organoids have created unprecedented possibilities for modeling human brain developmental diseases in vitro (141). We hope that continued investigations of the role of primary cilia in the developing cerebral cortex will lead to new understanding of neural cell function and communication. Moreover, these investigations will provide new ideas for the further diagnosis and treatment of ciliopathies.
Acknowledgments
We thank Qing-Yuan Tang for criticism of the article.
Funding: This study was supported by the “Deng Feng” Cross Innovation Program (No. EK112520180209).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-107/rc
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-107/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-107/coif). WZ serves as an unpaid Executive Editor-in-Chief of Pediatric Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 2007;8:880-93. [Crossref] [PubMed]
- Mitchison HM, Valente EM. Motile and non-motile cilia in human pathology: from function to phenotypes. J Pathol 2017;241:294-309. Correction in J Pathol 2017;241:564. [Crossref] [PubMed]
- Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci 1968;3:207-30. [Crossref] [PubMed]
- Bloodgood RA. From central to rudimentary to primary: the history of an underappreciated organelle whose time has come. The primary cilium. Methods Cell Biol 2009;94:3-52. [Crossref] [PubMed]
- Narita K, Kawate T, Kakinuma N, et al. Multiple primary cilia modulate the fluid transcytosis in choroid plexus epithelium. Traffic 2010;11:287-301. [Crossref] [PubMed]
- Braun DA, Hildebrandt F. Ciliopathies. Cold Spring Harb Perspect Biol 2017;9:a028191. [Crossref] [PubMed]
- DUNCAN D. Electron microscope study of the embryonic neural tube and notochord. Tex Rep Biol Med 1957;15:367-77. [PubMed]
- Fuchs JL, Schwark HD. Neuronal primary cilia: a review. Cell Biol Int 2004;28:111-8. [Crossref] [PubMed]
- Youn YH, Han YG. Primary Cilia in Brain Development and Diseases. Am J Pathol 2018;188:11-22. [Crossref] [PubMed]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 2010;11:331-44. [Crossref] [PubMed]
- Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep 2012;13:608-18. [Crossref] [PubMed]
- Wei Q, Ling K, Hu J. The essential roles of transition fibers in the context of cilia. Curr Opin Cell Biol 2015;35:98-105. [Crossref] [PubMed]
- Lee SH, Joo K, Jung EJ, et al. Export of membrane proteins from the Golgi complex to the primary cilium requires the kinesin motor, KIFC1. FASEB J 2018;32:957-68. [Crossref] [PubMed]
- Yamamoto M, Kataoka K. Electron microscopic observation of the primary cilium in the pancreatic islets. Arch Histol Jpn 1986;49:449-57. [Crossref] [PubMed]
- Cohen E, Meininger V. Ultrastructural analysis of primary cilium in the embryonic nervous tissue of mouse. Int J Dev Neurosci 1987;5:43-51. [Crossref] [PubMed]
- Sun S, Fisher RL, Bowser SS, et al. Three-dimensional architecture of epithelial primary cilia. Proc Natl Acad Sci U S A 2019;116:9370-9. [Crossref] [PubMed]
- Kiesel P, Alvarez Viar G, Tsoy N, et al. The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nat Struct Mol Biol 2020;27:1115-24. [Crossref] [PubMed]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol 1995;131:1517-27. [Crossref] [PubMed]
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol 2008;85:23-61. [Crossref] [PubMed]
- Yang TT, Tran MNT, Chong WM, et al. Single-particle tracking localization microscopy reveals nonaxonemal dynamics of intraflagellar transport proteins at the base of mammalian primary cilia. Mol Biol Cell 2019;30:828-37. [Crossref] [PubMed]
- Stepanek L, Pigino G. Microtubule doublets are double-track railways for intraflagellar transport trains. Science 2016;352:721-4. [Crossref] [PubMed]
- Taschner M, Lorentzen E. The Intraflagellar Transport Machinery. Cold Spring Harb Perspect Biol 2016;8:a028092. [Crossref] [PubMed]
- Hsiao YC, Tuz K, Ferland RJ. Trafficking in and to the primary cilium. Cilia 2012;1:4. [Crossref] [PubMed]
- Ishikawa H, Marshall WF. Intraflagellar Transport and Ciliary Dynamics. Cold Spring Harb Perspect Biol 2017;9:a021998. [Crossref] [PubMed]
- Pugacheva EN, Jablonski SA, Hartman TR, et al. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 2007;129:1351-63. [Crossref] [PubMed]
- Hubbert C, Guardiola A, Shao R, et al. HDAC6 is a microtubule-associated deacetylase. Nature 2002;417:455-8. [Crossref] [PubMed]
- Zhang YC, Bai YF, Yuan JF, et al. CEP55 promotes cilia disassembly through stabilizing Aurora A kinase. J Cell Biol 2021;220:e202003149. [Crossref] [PubMed]
- Phua SC, Chiba S, Suzuki M, et al. Dynamic Remodeling of Membrane Composition Drives Cell Cycle through Primary Cilia Excision. Cell 2017;168:264-279.e15. [Crossref] [PubMed]
- Ford MJ, Yeyati PL, Mali GR, et al. A Cell/Cilia Cycle Biosensor for Single-Cell Kinetics Reveals Persistence of Cilia after G1/S Transition Is a General Property in Cells and Mice. Dev Cell 2018;47:509-523.e5. [Crossref] [PubMed]
- Gerth-Kahlert C, Koller S. Ciliopathies. Klin Monbl Augenheilkd 2018;235:264-72. [PubMed]
- Rizzo JF 3rd, Berson EL, Lessell S. Retinal and neurologic findings in the Laurence-Moon-Bardet-Biedl phenotype. Ophthalmology 1986;93:1452-6. [Crossref] [PubMed]
- Baker K, Northam GB, Chong WK, et al. Neocortical and hippocampal volume loss in a human ciliopathy: A quantitative MRI study in Bardet-Biedl syndrome. Am J Med Genet A 2011;155A:1-8. [Crossref] [PubMed]
- Bennouna-Greene V, Kremer S, Stoetzel C, et al. Hippocampal dysgenesis and variable neuropsychiatric phenotypes in patients with Bardet-Biedl syndrome underline complex CNS impact of primary cilia. Clin Genet 2011;80:523-31. [Crossref] [PubMed]
- Keppler-Noreuil KM, Blumhorst C, Sapp JC, et al. Brain tissue- and region-specific abnormalities on volumetric MRI scans in 21 patients with Bardet-Biedl syndrome (BBS). BMC Med Genet 2011;12:101. [Crossref] [PubMed]
- Tsang SH, Aycinena ARP, Sharma T. Ciliopathy: Bardet-Biedl Syndrome. Adv Exp Med Biol 2018;1085:171-4. [Crossref] [PubMed]
- Azukizawa T, Yamamoto M, Narumiya S, et al. Oral-facial-digital syndrome type 1 with hypothalamic hamartoma and Dandy-Walker malformation. Pediatr Neurol 2013;48:329-32. [Crossref] [PubMed]
- Del Giudice E, Macca M, Imperati F, et al. CNS involvement in OFD1 syndrome: a clinical, molecular, and neuroimaging study. Orphanet J Rare Dis 2014;9:74. [Crossref] [PubMed]
- Bruel AL, Franco B, Duffourd Y, et al. Fifteen years of research on oral-facial-digital syndromes: from 1 to 16 causal genes. J Med Genet 2017;54:371-80. [Crossref] [PubMed]
- Horlenko O, Lenchenko A, Kossey G, et al. ORAL-FACIAL-DIGITAL SYNDROME TYPE I (CLINICAL CASE). Georgian Med News 2018;47-51. [PubMed]
- Moerman P, Verbeken E, Fryns JP, et al. The Meckel Syndrome. Pathological and cytogenetic observations in eight cases. Hum Genet 1982;62:240-5. [Crossref] [PubMed]
- Salonen R, Paavola P. Meckel syndrome. J Med Genet 1998;35:497-501. [Crossref] [PubMed]
- Kyttälä M, Tallila J, Salonen R, et al. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet 2006;38:155-7. [Crossref] [PubMed]
- Barker AR, Thomas R, Dawe HR. Meckel-Gruber syndrome and the role of primary cilia in kidney, skeleton, and central nervous system development. Organogenesis 2014;10:96-107. [Crossref] [PubMed]
- Sattar S, Gleeson JG. The ciliopathies in neuronal development: a clinical approach to investigation of Joubert syndrome and Joubert syndrome-related disorders. Dev Med Child Neurol 2011;53:793-8. [Crossref] [PubMed]
- Guo J, Otis JM, Suciu SK, et al. Primary Cilia Signaling Promotes Axonal Tract Development and Is Disrupted in Joubert Syndrome-Related Disorders Models. Dev Cell 2019;51:759-774.e5. [Crossref] [PubMed]
- Carter CS, Vogel TW, Zhang Q, et al. Abnormal development of NG2+PDGFR-α+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model. Nat Med 2012;18:1797-804. [Crossref] [PubMed]
- Wang L, Liu Y, Stratigopoulos G, et al. Bardet-Biedl syndrome proteins regulate intracellular signaling and neuronal function in patient-specific iPSC-derived neurons. J Clin Invest 2021;131:146287. [Crossref] [PubMed]
- Pezzella N, Bove G, Tammaro R, et al. OFD1: One gene, several disorders. Am J Med Genet C Semin Med Genet 2022;190:57-71. [Crossref] [PubMed]
- D'Angelo A, De Angelis A, Avallone B, et al. Ofd1 controls dorso-ventral patterning and axoneme elongation during embryonic brain development. PLoS One 2012;7:e52937. [Crossref] [PubMed]
- Hunkapiller J, Singla V, Seol A, et al. The ciliogenic protein Oral-Facial-Digital 1 regulates the neuronal differentiation of embryonic stem cells. Stem Cells Dev 2011;20:831-41. [Crossref] [PubMed]
- Hartill V, Szymanska K, Sharif SM, et al. Meckel-Gruber Syndrome: An Update on Diagnosis, Clinical Management, and Research Advances. Front Pediatr 2017;5:244. [Crossref] [PubMed]
- Goetz SC, Bangs F, Barrington CL, et al. The Meckel syndrome- associated protein MKS1 functionally interacts with components of the BBSome and IFT complexes to mediate ciliary trafficking and hedgehog signaling. PLoS One 2017;12:e0173399. [Crossref] [PubMed]
- Azevedo FA, Carvalho LR, Grinberg LT, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 2009;513:532-41. [Crossref] [PubMed]
- Andreu-Cervera A, Catala M, Schneider-Maunoury S. Cilia, ciliopathies and hedgehog-related forebrain developmental disorders. Neurobiol Dis 2021;150:105236. [Crossref] [PubMed]
- Sarkisian MR, Guadiana SM. Influences of primary cilia on cortical morphogenesis and neuronal subtype maturation. Neuroscientist 2015;21:136-51. [Crossref] [PubMed]
- Baldi P, Alhassen W, Chen S, et al. Large-scale analysis reveals spatiotemporal circadian patterns of cilia transcriptomes in the primate brain. J Neurosci Res 2021;99:2610-24. [Crossref] [PubMed]
- Chen S, Alhassen W, Vakil Monfared R, et al. Dynamic Changes of Brain Cilia Transcriptomes across the Human Lifespan. Int J Mol Sci 2021;22:10387. [Crossref] [PubMed]
- Gigante ED, Taylor MR, Ivanova AA, et al. ARL13B regulates Sonic hedgehog signaling from outside primary cilia. Elife 2020;9:50434. [Crossref] [PubMed]
- Higginbotham H, Guo J, Yokota Y, et al. Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat Neurosci 2013;16:1000-7. [Crossref] [PubMed]
- Higginbotham H, Eom TY, Mariani LE, et al. Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell 2012;23:925-38. [Crossref] [PubMed]
- Nakayama K, Katoh Y. Architecture of the IFT ciliary trafficking machinery and interplay between its components. Crit Rev Biochem Mol Biol 2020;55:179-96. [Crossref] [PubMed]
- Welburn JP, Cheeseman IM. The microtubule-binding protein Cep170 promotes the targeting of the kinesin-13 depolymerase Kif2b to the mitotic spindle. Mol Biol Cell 2012;23:4786-95. [Crossref] [PubMed]
- Zhang W, Yang SL, Yang M, et al. Modeling microcephaly with cerebral organoids reveals a WDR62-CEP170-KIF2A pathway promoting cilium disassembly in neural progenitors. Nat Commun 2019;10:2612. [Crossref] [PubMed]
- Gabriel E, Wason A, Ramani A, et al. CPAP promotes timely cilium disassembly to maintain neural progenitor pool. EMBO J 2016;35:803-19. [Crossref] [PubMed]
- Ding W, Wu Q, Sun L, et al. Cenpj Regulates Cilia Disassembly and Neurogenesis in the Developing Mouse Cortex. J Neurosci 2019;39:1994-2010. [Crossref] [PubMed]
- Garcez PP, Diaz-Alonso J, Crespo-Enriquez I, et al. Cenpj/CPAP regulates progenitor divisions and neuronal migration in the cerebral cortex downstream of Ascl1. Nat Commun 2015;6:6474. [Crossref] [PubMed]
- Meneses Iack P, Rayêe D, Lent R, et al. Microcephaly gene Cenpj regulates axonal growth in cortical neurons through microtubule destabilization. J Neurochem 2022;161:320-4. [Crossref] [PubMed]
- Ocbina PJ, Eggenschwiler JT, Moskowitz I, et al. Complex interactions between genes controlling trafficking in primary cilia. Nat Genet 2011;43:547-53. [Crossref] [PubMed]
- King CR. Fbxo41 Promotes Disassembly of Neuronal Primary Cilia. Sci Rep 2019;9:8179. [Crossref] [PubMed]
- Wiegering A, Rüther U, Gerhardt C. The ciliary protein Rpgrip1l in development and disease. Dev Biol 2018;442:60-8. [Crossref] [PubMed]
- Postel M, Karam A, Pézeron G, et al. A multiscale mathematical model of cell dynamics during neurogenesis in the mouse cerebral cortex. BMC Bioinformatics 2019;20:470. [Crossref] [PubMed]
- Tschaikner P, Enzler F, Torres-Quesada O, et al. Hedgehog and Gpr161: Regulating cAMP Signaling in the Primary Cilium. Cells 2020;9:118. [Crossref] [PubMed]
- Shimada IS, Somatilaka BN, Hwang SH, et al. Derepression of sonic hedgehog signaling upon Gpr161 deletion unravels forebrain and ventricular abnormalities. Dev Biol 2019;450:47-62. [Crossref] [PubMed]
- Huet D, Blisnick T, Perrot S, et al. The GTPase IFT27 is involved in both anterograde and retrograde intraflagellar transport. Elife 2014;3:e02419. [Crossref] [PubMed]
- Shohayeb B, Ho U, Yeap YY, et al. The association of microcephaly protein WDR62 with CPAP/IFT88 is required for cilia formation and neocortical development. Hum Mol Genet 2020;29:248-63. [Crossref] [PubMed]
- Willaredt MA, Hasenpusch-Theil K, Gardner HA, et al. A crucial role for primary cilia in cortical morphogenesis. J Neurosci 2008;28:12887-900. [Crossref] [PubMed]
- Pruski M, Hu L, Yang C, et al. Roles for IFT172 and Primary Cilia in Cell Migration, Cell Division, and Neocortex Development. Front Cell Dev Biol 2019;7:287. [Crossref] [PubMed]
- Plotnikova OV, Seo S, Cottle DL, et al. INPP5E interacts with AURKA, linking phosphoinositide signaling to primary cilium stability. J Cell Sci 2015;128:364-72. [PubMed]
- Hasenpusch-Theil K, Laclef C, Colligan M, et al. A transient role of the ciliary gene Inpp5e in controlling direct versus indirect neurogenesis in cortical development. Elife 2020;9:58162. [Crossref] [PubMed]
- Hu WF, Pomp O, Ben-Omran T, et al. Katanin p80 regulates human cortical development by limiting centriole and cilia number. Neuron 2014;84:1240-57. [Crossref] [PubMed]
- Akkaya C, Atak D, Kamacioglu A, et al. Roles of developmentally regulated KIF2A alternative isoforms in cortical neuron migration and differentiation. Development 2021;148:dev192674. [Crossref] [PubMed]
- Poirier K, Lebrun N, Broix L, et al. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat Genet 2013;45:639-47. [Crossref] [PubMed]
- Chen JL, Chang CH, Tsai JW. Gli2 Rescues Delays in Brain Development Induced by Kif3a Dysfunction. Cereb Cortex 2019;29:751-64. [Crossref] [PubMed]
- Abdelhamed ZA, Abdelmottaleb DI, El-Asrag ME, et al. The ciliary Frizzled-like receptor Tmem67 regulates canonical Wnt/β-catenin signalling in the developing cerebellum via Hoxb5. Sci Rep 2019;9:5446. [Crossref] [PubMed]
- Shim JW, Territo PR, Simpson S, et al. Hydrocephalus in a rat model of Meckel Gruber syndrome with a TMEM67 mutation. Sci Rep 2019;9:1069. [Crossref] [PubMed]
- Valente EM, Logan CV, Mougou-Zerelli S, et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet 2010;42:619-25. [Crossref] [PubMed]
- Edvardson S, Shaag A, Zenvirt S, et al. Joubert syndrome 2 (JBTS2) in Ashkenazi Jews is associated with a TMEM216 mutation. Am J Hum Genet 2010;86:93-7. [Crossref] [PubMed]
- Mukhopadhyay S, Wen X, Chih B, et al. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev 2010;24:2180-93. [Crossref] [PubMed]
- Nakagawa N, Li J, Yabuno-Nakagawa K, et al. APC sets the Wnt tone necessary for cerebral cortical progenitor development. Genes Dev 2017;31:1679-92. [Crossref] [PubMed]
- Laclef C. Primary cilia control different steps of brain development. Med Sci (Paris) 2014;30:980-90. [Crossref] [PubMed]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009;32:149-84. [Crossref] [PubMed]
- Hasenpusch-Theil K, Theil T. The Multifaceted Roles of Primary Cilia in the Development of the Cerebral Cortex. Front Cell Dev Biol 2021;9:630161. [Crossref] [PubMed]
- Wilsch-Bräuninger M, Peters J, Paridaen JT, et al. Basolateral rather than apical primary cilia on neuroepithelial cells committed to delamination. Development 2012;139:95-105. [Crossref] [PubMed]
- Pan J, Snell W. The primary cilium: keeper of the key to cell division. Cell 2007;129:1255-7. [Crossref] [PubMed]
- Paridaen JT, Wilsch-Bräuninger M, Huttner WB. Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell 2013;155:333-44. [Crossref] [PubMed]
- Tang CJ, Fu RH, Wu KS, et al. CPAP is a cell-cycle regulated protein that controls centriole length. Nat Cell Biol 2009;11:825-31. [Crossref] [PubMed]
- Lin YN, Lee YS, Li SK, et al. Loss of CPAP in developing mouse brain and its functional implication for human primary microcephaly. J Cell Sci 2020;133:jcs243592. [Crossref] [PubMed]
- Guerreiro A, De Sousa F, Liaudet N, et al. WDR62 localizes katanin at spindle poles to ensure synchronous chromosome segregation. J Cell Biol 2021;220:e202007171. [Crossref] [PubMed]
- Gabriel E, Ramani A, Altinisik N, et al. Human Brain Organoids to Decode Mechanisms of Microcephaly. Front Cell Neurosci 2020;14:115. [Crossref] [PubMed]
- Tong CK, Han YG, Shah JK, et al. Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci U S A 2014;111:12438-43. [Crossref] [PubMed]
- Wiegering A, Dildrop R, Kalfhues L, et al. Cell type-specific regulation of ciliary transition zone assembly in vertebrates. EMBO J 2018;37:e97791. [Crossref] [PubMed]
- Humbert MC, Weihbrecht K, Searby CC, et al. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci U S A 2012;109:19691-6. [Crossref] [PubMed]
- Evsyukova I, Plestant C, Anton ES. Integrative mechanisms of oriented neuronal migration in the developing brain. Annu Rev Cell Dev Biol 2013;29:299-353. [Crossref] [PubMed]
- Métin C. Cilia and neuronal migrations. Med Sci (Paris) 2014;30:991-5. [Crossref] [PubMed]
- Guo J, Higginbotham H, Li J, et al. Developmental disruptions underlying brain abnormalities in ciliopathies. Nat Commun 2015;6:7857. [Crossref] [PubMed]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci 2004;27:392-9. [Crossref] [PubMed]
- Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell 2007;128:29-43. [Crossref] [PubMed]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, et al. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci 2010;30:1582-94. [Crossref] [PubMed]
- Li G, Adesnik H, Li J, et al. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci 2008;28:1085-98. [Crossref] [PubMed]
- Baudoin JP, Viou L, Launay PS, et al. Tangentially migrating neurons assemble a primary cilium that promotes their reorientation to the cortical plate. Neuron 2012;76:1108-22. [Crossref] [PubMed]
- Luccardini C, Hennekinne L, Viou L, et al. N-cadherin sustains motility and polarity of future cortical interneurons during tangential migration. J Neurosci 2013;33:18149-60. [Crossref] [PubMed]
- Luccardini C, Leclech C, Viou L, et al. Cortical interneurons migrating on a pure substrate of N-cadherin exhibit fast synchronous centrosomal and nuclear movements and reduced ciliogenesis. Front Cell Neurosci 2015;9:286. [Crossref] [PubMed]
- He CW, Liao CP, Pan CL. Wnt signalling in the development of axon, dendrites and synapses. Open Biol 2018;8:180116. [Crossref] [PubMed]
- Kumamoto N, Gu Y, Wang J, et al. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci 2012;15:399-405, S1.
- Guadiana SM, Semple-Rowland S, Daroszewski D, et al. Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. J Neurosci 2013;33:2626-38. [Crossref] [PubMed]
- Ishii S, Sasaki T, Mohammad S, et al. Primary cilia safeguard cortical neurons in neonatal mouse forebrain from environmental stress-induced dendritic degeneration. Proc Natl Acad Sci U S A 2021;118:e2012482118. [Crossref] [PubMed]
- May-Simera HL, Kelley MW. Cilia, Wnt signaling, and the cytoskeleton. Cilia 2012;1:7. [Crossref] [PubMed]
- Bangs F, Anderson KV. Primary Cilia and Mammalian Hedgehog Signaling. Cold Spring Harb Perspect Biol 2017;9:a028175. [Crossref] [PubMed]
- Anvarian Z, Mykytyn K, Mukhopadhyay S, et al. Cellular signalling by primary cilia in development, organ function and disease. Nat Rev Nephrol 2019;15:199-219. [Crossref] [PubMed]
- Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol 2013;14:416-29. [Crossref] [PubMed]
- Eguether T, San Agustin JT, Keady BT, et al. IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev Cell 2014;31:279-90. [Crossref] [PubMed]
- Desai PB, Stuck MW, Lv B, et al. Ubiquitin links smoothened to intraflagellar transport to regulate Hedgehog signaling. J Cell Biol 2020;219:e201912104. [Crossref] [PubMed]
- Le TL, Sribudiani Y, Dong X, et al. Bi-allelic Variations of SMO in Humans Cause a Broad Spectrum of Developmental Anomalies Due to Abnormal Hedgehog Signaling. Am J Hum Genet 2020;106:779-92. [Crossref] [PubMed]
- Mukhopadhyay S, Wen X, Ratti N, et al. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell 2013;152:210-23. [Crossref] [PubMed]
- Pal K, Mukhopadhyay S. Primary cilium and sonic hedgehog signaling during neural tube patterning: role of GPCRs and second messengers. Dev Neurobiol 2015;75:337-48. [Crossref] [PubMed]
- Roberts AJ. Emerging mechanisms of dynein transport in the cytoplasm versus the cilium. Biochem Soc Trans 2018;46:967-82. [Crossref] [PubMed]
- Legué E, Liem KF Jr. Mutations in Ciliary Trafficking Genes affect Sonic Hedgehog-dependent Neural Tube Patterning Differentially along the Anterior-Posterior Axis. Neuroscience 2020;450:3-14. [Crossref] [PubMed]
- Boitard M, Bocchi R, Egervari K, et al. Wnt signaling regulates multipolar-to-bipolar transition of migrating neurons in the cerebral cortex. Cell Rep 2015;10:1349-61. [Crossref] [PubMed]
- Oh EC, Katsanis N. Context-dependent regulation of Wnt signaling through the primary cilium. J Am Soc Nephrol 2013;24:10-8. [Crossref] [PubMed]
- Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development 2009;136:3089-98. [Crossref] [PubMed]
- Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS One 2009;4:e6839. [Crossref] [PubMed]
- Louvi A, Grove EA. Cilia in the CNS: the quiet organelle claims center stage. Neuron 2011;69:1046-60. [Crossref] [PubMed]
- Park SM, Lim JS, Ramakrishina S, et al. Brain Somatic Mutations in MTOR Disrupt Neuronal Ciliogenesis, Leading to Focal Cortical Dyslamination. Neuron 2018;99:83-97.e7. [Crossref] [PubMed]
- Foerster P, Daclin M, Asm S, et al. mTORC1 signaling and primary cilia are required for brain ventricle morphogenesis. Development 2017;144:201-10. [PubMed]
- Baas AF, Boudeau J, Sapkota GP, et al. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J 2003;22:3062-72. [Crossref] [PubMed]
- Dang LT, Vaid S, Lin G, et al. STRADA-mutant human cortical organoids model megalencephaly and exhibit delayed neuronal differentiation. Dev Neurobiol 2021;81:696-709. [Crossref] [PubMed]
- Cullen CL, O'Rourke M, Beasley SJ, et al. Kif3a deletion prevents primary cilia assembly on oligodendrocyte progenitor cells, reduces oligodendrogenesis and impairs fine motor function. Glia 2021;69:1184-203. [Crossref] [PubMed]
- Sipos É, Komoly S, Ács P. Quantitative Comparison of Primary Cilia Marker Expression and Length in the Mouse Brain. J Mol Neurosci 2018;64:397-409. [Crossref] [PubMed]
- Tabata H, Nagata K. Decoding the molecular mechanisms of neuronal migration using in utero electroporation. Med Mol Morphol 2016;49:63-75. [Crossref] [PubMed]
- Adams JW, Cugola FR, Muotri AR. Brain Organoids as Tools for Modeling Human Neurodevelopmental Disorders. Physiology (Bethesda) 2019;34:365-75. [Crossref] [PubMed]
- Shou Y, Liang F, Xu S, et al. The Application of Brain Organoids: From Neuronal Development to Neurological Diseases. Front Cell Dev Biol 2020;8:579659. [Crossref] [PubMed]
Cite this article as: Peng T, Cheng Y, Xiong M, Zhou WH, Cheng GQ. Primary cilia in the development of the cerebral cortex: a literature review. Pediatr Med 2023;6:26.

