
Bronchopulmonary dysplasia (BPD): a change in perspective
About 50 years ago, Northway was first to describe the entity of bronchopulmonary dysplasia (BPD) (1). His description of a syndromic disease of neonates below 2 kg of birthweight, who develop pulmonary fibrosis and pulmonary emphysema caused by severe respiratory distress treated with conventional ventilation and 100% oxygen, is today considered as “old” BPD. BPD together with asthma and cystic fibrosis represents the three most common entities of chronic lung disease of infants in the western world that continue into adulthood. For asthma and BPD incidences are increasing, for BPD—at least in the most immature infants—severity is increasing, too. Another interesting common fact is that both, BPD and asthma, may be defined as phenotypes induced by different etiologies. For BPD, several corner stones changing in neonatal medical care caused an increase in incidence and severity. Improved survival of tiny premature neonates; the establishment of specialized care to enable spontaneous ventilation supported by continuous positive airway pressure (CPAP); the reduction of perinatal inflammation and infection of mothers and fetus/infant by the use of antibiotics and anti-inflammatory drugs (e.g., steroids). For steroids in turn, a new role was invented which was the induction of fetal lung maturation by prenatal treatment of mothers. Finally, the ability to treat the immature surfactant deficient lung with intratracheal pulmonary surfactant was a significant step that additionally improved perinatal care and reduced morbidity and mortality of preterm neonates, including a reduction of the (old) BPD originally described by Northway. Today millions of premature infants have been treated with surfactant since the first medical report of Fujiwara 1980 (2).
However, BPD did not disappear, rather was changed in definition (3,4). Thus, a new phenotype of BPD of more premature infants born below 28 weeks of postmenstrual age appeared. Husain et al. characterized this new BPD pathologically by a partial or total arrest of alveolar development (5). The pathophysiology of BPD includes several non-physiologic hits on the developing premature lung whose function is launched prematurely (6) (Figure 1).
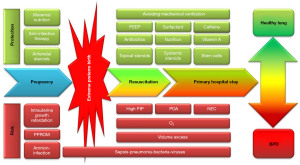
The “new BPD” phenotype develops during interference with the continuing lung development induced by inflammation, oxidative stress, ventilator induced damage, “microbiologic stress” and genetic predisposition. Since these hits may be at different time points with regard to development stages, of different (I) severity, e.g., sepsis or pneumonia, and (II) quality, e.g., ventilation trauma, bacterial, viral or fungal infection, the “multiple hit” etiology hypothesis makes clear that there will be no simple solutions (“no silver bullet for prevention of BPD”). In addition, prenatal and genetic causes of BPD are described as modifiers of the disease (8). Together, these factors may lead to sustained disrupted alveolar and microvascular development (9), and finally, to disability to achieve the full airway growth potential in adulthood (10). Follow-up studies could show progressive obstructive pulmonary disease after extreme prematurity even after introduction of surfactant treatment (11). Additional long-term observations suggest similarities of BPD in adulthood with chronic obstructive pulmonary disease (COPD) in adulthood (12,13).
Today, we haven’t fully understood the pathophysiological mechanisms behind BPD; we have no chance to give a causal therapy to premature born infants and scarce possibilities to avoid preterm birth potentially resulting in BPD.
The course of the disease may be insidiously, showing improvement during the first years of life after a severe course during initial hospital stay. Yet, we have better understood, that previously extremely preterm infants with BPD develop progressive decreasing lung function during early adulthood (14). Recently, the European Respiratory Society suggested guidelines for long term follow-up of infants with BPD and contextualized it as a new group of COPD (15). Today’s long-term follow-up of BPD is insufficient and multidisciplinary follow-up is needed since it is often accompanied by neurodevelopmental delay and/or cerebral palsy. While adult medicine gradually has to learn about role of premature birth for diseases later in life, neonatologists already understood important corner stones of BPD due to 50 years of experience: An early lung protective treatment strategy during the primary hospital stay and especially during the first days of life is of key importance to improve the outcome of the disease.
In their review in this journal, Verder et al. highlight the most recent pathophysiologic findings, early prediction and treatment strategies of BPD based upon papers published predominantly during the past 15 years (16), as since Northway’s initial report, the understanding of BPD has expanded in terms of course of the disease and etiology. Considering this, e.g., new aspects as pulmonary microbiome or precise differentiation of etiologic factors that nowadays are known for contributing to the pathophysiology of BPD have to be taken into account for development of early diagnosis and early prevention and/or treatment strategies. Consequently, Verder and colleagues concentrate in their review on the first 28 days of live of premature neonates at risk. The authors develop the hypothesis that by early management and use of predictive tools early selective preventive treatments can be initiated. However, those patients remaining with BPD beyond the first 4 weeks of life also need to be counseled, since obstruction and avoidable factors like smoking contribute to the course of the disease (17). Early viral infections in infancy like respiratory syncytial virus seem to be disease modifiers as well (18).
In conclusion, the perspective of BPD has changed and widened. Due to improved survival, the disease approaches the clinical field of adult pulmonologists with a new similar phenotype to COPD. And patients need to be counseled at each stage of life due to the chronic character of the disease. Thus, early reliable tools for BPD prediction may pave a promising way for early BPD-preventive treatments. Adult pulmonologists seeing patients with COPD like symptoms already in the 3rd decade of life will have to add the questions about gestational age and/or birth weight to their repertoire.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Pediatric Medicine. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-22-53/coif). GS received speaking honoraria by Chiesi, Hamburg, Germany. EH reported that he has had patent on less invasive surfactant therapy, participated in Vitamin A study, Neovit A, RSV, European Group, and served as an unpaid board member of German Research Council (DFG) and Quality in Neonatal Medicine (IQTIG). EH received speaking fees and travel support from Chiesi Pharmaceuticals, a surfactant producer. EH serves as an unpaid editorial board member of Pediatric Medicine from December 2022 to November 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967;276:357-68. [Crossref] [PubMed]
- Fujiwara T, Maeta H, Chida S, et al. Artificial surfactant therapy in hyaline-membrane disease. Lancet 1980;1:55-9. [Crossref] [PubMed]
- Walsh MC, Yao Q, Gettner P, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114:1305-11. [Crossref] [PubMed]
- Jensen EA, Dysart K, Gantz MG, et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am J Respir Crit Care Med 2019;200:751-9. [Crossref] [PubMed]
- Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 1998;29:710-7. [Crossref] [PubMed]
- Day CL, Ryan RM. Bronchopulmonary dysplasia: new becomes old again! Pediatr Res 2017;81:210-3. [Crossref] [PubMed]
- Collins JJP, Tibboel D, de Kleer IM, et al. The Future of Bronchopulmonary Dysplasia: Emerging Pathophysiological Concepts and Potential New Avenues of Treatment. Front Med (Lausanne) 2017;4:61. [Crossref] [PubMed]
- Hamvas A, Feng R, Bi Y, et al. Exome sequencing identifies gene variants and networks associated with extreme respiratory outcomes following preterm birth. BMC Genet 2018;19:94. [Crossref] [PubMed]
- Thébaud B, Goss KN, Laughon M, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers 2019;5:78. [Crossref] [PubMed]
- Moschino L, Bonadies L, Baraldi E. Lung growth and pulmonary function after prematurity and bronchopulmonary dysplasia. Pediatr Pulmonol 2021;56:3499-508. [Crossref] [PubMed]
- Doyle LW, Irving L, Haikerwal A, et al. Airway obstruction in young adults born extremely preterm or extremely low birth weight in the postsurfactant era. Thorax 2019;74:1147-53. [Crossref] [PubMed]
- Rennard SI, Drummond MB. Early chronic obstructive pulmonary disease: definition, assessment, and prevention. Lancet 2015;385:1778-88. [Crossref] [PubMed]
- Um-Bergström P, Pourbazargan M, Brundin B, et al. Increased cytotoxic T-cells in the airways of adults with former bronchopulmonary dysplasia. Eur Respir J 2022;60:2102531. [Crossref] [PubMed]
- Kotecha SJ, Gibbons JTD, Course CW, et al. Geographical Differences and Temporal Improvements in Forced Expiratory Volume in 1 Second of Preterm-Born Children: A Systematic Review and Meta-analysis. JAMA Pediatr 2022;176:867-77. [Crossref] [PubMed]
- Duijts L, van Meel ER, Moschino L, et al. European Respiratory Society guideline on long-term management of children with bronchopulmonary dysplasia. Eur Respir J 2020;55:1900788. [Crossref] [PubMed]
- Verder H, Li ZK, Ramanathan R, et al. Bronchopulmonary dysplasia with focus on early prediction and treatment: a narrative review. Pediatr Med 2023;6:13.
- Bui DS, Perret JL, Walters EH, et al. Association between very to moderate preterm births, lung function deficits, and COPD at age 53 years: analysis of a prospective cohort study. Lancet Respir Med 2022;10:478-84. [Crossref] [PubMed]
- Townsi N, Laing IA, Hall GL, et al. The impact of respiratory viruses on lung health after preterm birth. Eur Clin Respir J 2018;5:1487214. [Crossref] [PubMed]
Cite this article as: Stichtenoth G, Herting E. Bronchopulmonary dysplasia (BPD): a change in perspective. Pediatr Med 2023;6:11.

