Pharmacokinetics of drugs: newborn perspective
Introduction
“Children are not small adults” is true for the pediatric population and similarly “Neonates are not just small children” stands for newborns. These statements are significant with regards to the pharmacokinetics of drugs in neonates (1-3). Adult population pharmacokinetics principles cannot be extrapolated to neonates because of considerable inter and intraindividual variability resulting from differing gestational age, postnatal maturation, and rapid changes in physiology with development (4,5). Impaired cerebral growth after dexamethasone and reduced number of glomeruli following exposure to nephrotoxic drugs during nephrogenesis in preterm neonates is a reflection of such population-specific vulnerability (6-8).
Neonates undergo rapid and significant maturation and development, leading to changes in body composition, expression of drug-metabolizing enzymes, fluid distribution, and variable hepato-renal function. This can be further complicated by pathological conditions (such as growth restriction, sepsis, associated cardiomyopathy, and organ failure) or administered treatment (e.g., co-medication, surgical intervention, extracorporeal membrane oxygenation) (9,10). Adult drug doses, even after adjustment for body weight can be risky in neonates as it may expose this vulnerable population to potentially toxic doses and adverse drug reactions. Even now, approximately 65% of the drugs used in the neonatal intensive care units (NICUs) are still off-label and need dose optimization before administration (11). Therapeutic drug monitoring (TDM) may be helpful for clinical efficacy and to prevent therapeutic misadventures. Consequently, there is an obvious need to focus on the covariates contributing to this variability within this vulnerable population and adopt TDM for safe and efficacious drug administration whenever feasible (11).
Pharmacokinetics
Pharmacokinetics in simple terms refers to “what the body does to drugs”. This involves how the drugs are processed and disposed of via a series of processes, which involves: drug absorption, distribution, metabolism, and elimination (ADME) (10,12,13). The efficacy of the processes depends on functional maturity and may differ significantly with ontogeny and disease states of neonates. These processes contribute to significant differences in therapeutic efficacy which is observed between neonatal and adult populations (14).
Factors involved in pharmacokinetics
Drug absorption and bioavailability
The absorption of a drug refers to its translocation from the administered site into the systemic circulation. The absorption of a drug is influenced by multiple factors, including the dose, route of administration, carrier-mediated transport, molecular weight, lipid solubility, degree of ionization, and metabolism of the drug. Gestational age at birth, postnatal age, and developmental changes in neonates, add to the complexity of the process (12).
Intravascular drugs have rapid and better systemic availability compared to the ones administered from extravascular sites. The portion of a dose that enters the systemic circulation intact is defined as drug bioavailability. Delayed absorption may affect drug bioavailability and predicted peak concentrations, leading to a less than desired response (15).
Factors influencing drug absorption as based on the route of administration are described below:
Enteral absorption
The bioavailability of medications from enteral routes is determined by gastric acidity and emptying, gastrointestinal (GI) secretions, transport, first-pass metabolism, and bacterial flora (16). These also affect the time to achieve maximal plasma level of drugs (13). Many neonates tend to regurgitate orally administered drugs intermittently some times during therapy, which necessitates readministration. Moreover, many infants especially extreme premature infants are not on significant feeds in the initial few days of life, precluding the use of oral drugs in this population.
Role of Gastric pH
Gastric pH plays a significant role in the absorption of drugs. In term neonates, gastric pH transitions from an alkaline pH (pH 6–8) at birth to more acidic levels (pH 1–3) over the first 24–48 h of life (17-19). This change is transient, as gastric acid production declines. By the age of 2 years, pH is again acidic 1–3 similar to adult pH (19,20). This process is altered in preterm neonates with significant attenuation in gastric acid production until 2–3 weeks after birth (21). Enteral feeding may further reduce the gastric pH. Consequently, stomach emptying is delayed and there is decreased ionization of drugs, leading to increased absorption of weakly basic drugs such as penicillin G, amoxicillin, nafcillin, and erythromycin (22-24) and decreased absorption for acidic drugs such as phenobarbital and phenytoin (25).
Gastrointestinal secretions
At birth there is an immature pancreatic exocrine and biliary system (26), leading to insufficient synthesis of bile salts and lipase (27,28). Inefficient solubilization and intraluminal hydrolysis lead to reduced absorption and bioavailability of lipophilic compounds such as fat-soluble vitamins. However, these functions develop rapidly in the postnatal period (29).
Gastrointestinal emptying
Emptying times and intestinal motility are functions of postconceptional and postnatal age and affect drug absorption (30). Neonates exhibit relatively delayed gastric emptying compared to adults (31). Thus, gastric emptying times are prolonged in more premature infants.
Although feeding stimulates the development of gastric motility, feed composition also affects gastric emptying (32). For example, consumption of human milk and low-calorie formulas increases the rate of gastric emptying in contrast to the formulas containing higher caloric density and long-chain fatty acids (33,34).
Gastrointestinal transport
Carrier-mediated uptake systems in the gastrointestinal mucosa are crucial for efficient absorption. An immature transport function may reduce the bioavailability of medications (35). On the other hand, some transport proteins [e.g., multidrug resistance 1 (MDR1)] promote the extrusion of drugs from enterocytes into the gastrointestinal lumen, thereby decreasing their bioavailability (36). The intestinal influx oligopeptide transporter peptide transporter 1 (PEPT1) mRNA expression in neonates is marginally lower (0.8-fold) than older children (37). Expression of these transporter proteins improves with increasing postnatal age. Neonatal intestinal expressions of MDR1 and multidrug resistance protein 2 (MRP2) are similar to that in adults (38). Inconsistent drug absorption can also occur with congenital pathological processes (e.g., duodenal atresia) or surgical interventions (e.g., short bowel syndromes), which lead to deficient intestinal transporters like PEPT1 (39).
Gastrointestinal first-pass effects
Drug metabolism by gastrointestinal and hepatic enzymes is described as the “First-pass effect”. This leads to a reduction of the unmetabolized drug fraction reaching the systemic circulation and consequently alters predictable drug levels in the systemic circulation. The liver is the primary organ involved. Many drugs such as propranolol and morphine, undergo biotransformation in the liver (15). Some drugs, such as midazolam, are metabolized in the intestine, resulting in a diminished proportion of drugs reaching the systemic circulation (40). Enteral drug first-pass effect increases with gestational and postnatal maturation of metabolic pathways and their transport functions. Some drugs may be affected less by first-pass metabolism in immature neonates, thus increasing the proportion of unmetabolized drugs reaching systemic circulation (41). Thus, maturational changes results in erratic pharmacokinetic drug profiles in newborns.
Gastrointestinal bacterial flora
Bacterial flora influences intestinal motility and metabolism of a drug, playing an important role in its absorption (42,43). The ileum and colon are the primary concentration sites of bacterial flora. Immature gastrointestinal metabolic reactions and ineffective gastrointestinal first-pass metabolism results in improved oral bioavailability in neonates. It takes approximately four years for an infant to develop a mature, adult-like bacterial flora (42,44). This contributes to substantial inter-and intra-individual variability in oral bioavailability.
Genetic factors and underlying pathophysiology (endocrinopathies, gastrointestinal disorders, central nervous system disorders, and metabolic derangements), concomitant administration of drugs also contribute to variable enteral absorption. In summary, enteral bioavailability is influenced by a multitude of factors like postnatal developmental in a newborn, gestation specific factors, and underlying pathophysiology. Knowledge of differences in enteral bioavailability is essential to understand and guide dose adjustment for orally administered medications. This also explains the reliance on intravascular route for administration of critical medications such as antibiotics in neonates.
Non-enteral route of absorption
Drugs administered through non-enteral routes such as sublingual, intramuscular, subcutaneous, inhalation, and intravascular have an associated variability in absorption, depending on the route and developmental differences.
The sublingual route is highly variable in neonates and is not preferred because of uncertain and uncontrolled absorption. The intramuscular absorption is also reduced in neonates because of poor regional blood supply and decreased muscle mass. Lipophilic drugs, which rapidly diffuse into the capillaries, are an exception to this. There is also a concern of some drugs leading to adverse local tissue reactions and injury like calcium gluconate. Thus, these routes are rarely utilized except for a few drugs such as Vitamin K, aminoglycosides, and cephalosporin administration (45,46).
Drugs administered through the subcutaneous route can also have unpredictable systemic bioavailability because of multiple factors including a thin epidermis, reduced stratum corneum, a high degree of hydration, and the higher body surface area ratio of a preterm infant. These may aid in increased percutaneous absorption, while poor skin perfusion may reduce absorption (2,3). Both theophylline and caffeine have been successfully administered via topical gel, with the achievement of therapeutic serum drug concentrations. However, the inadvertent toxicity after percutaneous drug absorption is often overlooked. Historically, boric acid, hexachlorophene, alcohol, and corticosteroids toxicities have been reported after percutaneous administration (47,48).
Rectal absorption is less predictable in newborns than in older children and adults due to variable GI motility and drug insertion depth. The first-pass effect is more apparent with deeper administration of drugs, as the superior rectal vein drains into the portal vein via the inferior mesenteric vein. Drugs administered into the lower rectum have more bioavailability as the lower and middle rectal veins drain directly into the systemic circulation via the inferior vena cava bypassing the liver. Acetaminophen and diazepam have been traditionally administered by this route (48,49).
Inhalational and endotracheal administration also have uncontrolled absorption and greater systemic effects, increasing the potential for toxic exposure in neonates. Efficacy is also unpredictable by this route. Drugs such as epinephrine, lidocaine, atropine, and naloxone are administered by the endotracheal route in special circumstances, such as during resuscitation while intravenous access is established. Drugs such as surfactant and inhaled nitric oxide are administered through the intratracheal route for local action on the lungs. Bronchodilators and some steroids are used by inhalation for action at local site (i.e., lungs) and to potentially minimize systemic side effects (50).
Bioavailability after intravenous administration is not always complete. Although this route eliminates site-specific variables for absorption, rapid delivery of the drug after intravenous administration may result in rapid first-pass metabolism, especially when administered into low-lying umbilical venous catheters or peripheral sites in the lower extremities. Thus, a reduced amount of active drug reaches the systemic circulation and the target organ(s). Midazolam, morphine, and propranolol are especially susceptible to first pass pre-systemic clearance (7,8).
Drug distribution
Drug distribution refers to the process of movement of a drug through the body, from central circulation to various body compartments. Many factors, such as the pH, size and composition of the compartment, lipid solubility, plasma protein levels, tissue protein binding, membrane permeability, regional blood flow, and hemodynamic status affect the movement of a drug (from intravascular to an extravascular compartment) (48).
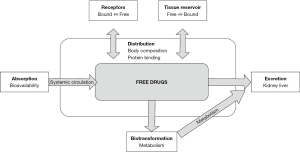
The intravascular space is the central compartment from where the drug is distributed at a faster rate to the heart, kidney, and liver, but at a slower rate to the brain, fatty tissue, and muscles. Drugs traverse back from the peripheral to the central compartment for elimination. Drug distributions occur until an equilibrium is established between an unbound fraction of the drug in the plasma and tissue fluids, as illustrated by Figure 1. It is influenced by numerous factors such as volume of distribution, body composition, tissue perfusion, and protein binding (51).
The volume of distribution (Vd)
The ‘apparent volume of distribution’ for a drug is defined as the hypothetical fluid volume that would accommodate all the drug in the body. It is calculated by dividing the total amount of drug given by the concentration of drug in the plasma (Eq. [1]) and is a useful marker to assess age-related changes in drug distribution.
Vd is a useful parameter for calculating a patient-specific loading dose. It is not similar to blood volume or physiological fluid compartments. It is the hypothetical volume of all the compartments through which a drug is dispersed. Understanding of contributing factors that lead to variation in Vd helps optimize dosages to achieve the desired peak concentration (Cmax) in a newborn. Developmental changes also affect Vd. It may be a function of postnatal enhancements in cardiac output, organ blood flow, tissue perfusion, changes in membrane permeabilities, maturation of carrier-mediated transport systems, and changes in tissue binding affinities since newborns and young infants have significantly greater liver, kidney, and brain masses relative to total body mass (48,52-54). Neonatal conditions, such as pulmonary hypertension, hypoplastic left heart, and patent ductus arteriosus, may affect perfusion and restrict the movement and distribution of a drug to the site of action, ultimately affecting the drug activity and efficacy (48,52-54).
Body composition
Body composition is another key factor in drug distribution. Body composition (total body water, total protein, and fat content) changes with gestational and postnatal age, which may produce significant quantitative changes in the Vd and plasma concentration.
As the fetus matures from 24 weeks of gestation to term, the percentage of total body water decreases from 85% to 75%, and the fat tissue increases from 1% to 16% (10,15,52,55). Body composition is also affected by the intrauterine environment and fetal disease states. Fat stores are abundant in infants born to women with diabetes but are reduced in infants with intrauterine growth restriction. These differences in body composition directly affect the dose of a drug required to affect a response. In comparison to adults, neonates have proportionally more body water than fat and have greater Vd for water-soluble compounds than fat-soluble compounds. Higher Vd has a lower drug peak concentration. Hydrophilic drugs like gentamicin have increased Vd especially in preterms (with high body water content), therefore a higher dose is required to achieve the same peak concentration compared with term neonates (56).
Protein binding
Only the ‘unbound drug’ (Free drug) is pharmacologically active, thus protein binding plays a significant role in the pharmacokinetics of a drug and its distribution. Newborns exhibit lower protein binding affinities for certain drugs such as penicillin, phenobarbital, phenytoin, and theophylline (57,58). The Vd increases with decreased protein binding. However, in the instance of reduced binding, for a given total serum concentration, there is a higher free fraction of available drug. An increased free fraction of the drug may result in an exaggerated therapeutic or toxic effect. This increased concentration of the drug is short-lived as the unbound form, or active drug has a higher clearance rate and is excreted at a faster pace. This difference in protein binding underscores the importance of measuring total and free serum drug concentrations in neonates (48).
Preterm infants have proteins with diverse qualities and quantities when compared to term neonates. They have a lower concentration of binding proteins such as albumin, lipoproteins, alpha-1-acid glycoprotein, and beta globulins, increased fetal albumin which has decreased affinity for drugs, lower plasma pH which hinders the binding of acidic drugs, and marked quantities of binding protein inhibitors. Thus, preterm infants have inconsistent responses to protein binding drugs when compared to term infants.
Pathological conditions
Pathological conditions can also affect the protein binding affinity and volume of distribution. Hepatic and renal diseases and hypoproteinaemia secondary to malnutrition, cystic fibrosis, burns, surgery, trauma, and acidosis decreases the plasma protein-drug binding due to decreased protein synthesis or the presence of competitive inhibitors. Hyperbilirubinemia also reduces the binding of acidic drugs like ampicillin, penicillin, phenobarbital, and phenytoin (57,59). On the contrary, ceftriaxone and sulphonamides lead to an increased amount of displaced bilirubin, which increases the risk of bilirubin encephalopathy (60,61).
Drug metabolism/biotransformation
Biotransformation is the process of metabolism of drugs within the body. It alters the chemical composition, converting lipophilic drugs into more polar hydrophilic derivatives to facilitate excretion or weaken the drug activity by turning it into a pharmacologically inactive form (62-65). The polar metabolites are generally inactive, but some can still retain pharmacological activity, such as theophylline and valganciclovir. They can also be toxic, as seen with N-acetyl-p-benzoquinone imine, a by-product of acetaminophen metabolism. Premature infants have reduced biotransformation capacity, because of incomplete organ development and consequent immature physiology. Factors such as reduced cellular uptake of drugs, lower hepatic enzyme capacity, decreased hepatic blood flow, and reduced biliary excretion are responsible for decreased metabolic activity and differences in biotransformation between a term and a pre-term infant (15,48). For example, the half-life of phenobarbital is longer in preterm infants than in term infants due to immaturity of phenobarbitone metabolism in preterms (66-68).
Gestational and postnatal development, environmental, and genetic factors also influence drug metabolism and biotransformation. Drug accumulation and exaggerated pharmacological response ensue if proactive modifications in the drug therapy are not initiated.
Although most of the biotransformation occurs in the liver, other organs, such as the kidney, intestine, skin, lungs, and adrenals often contribute. Metabolism in the liver starts with uptake by the hepatocytes. Once in hepatocytes, the next step involves either phase I and/or phase II reactions (64).
Phase I and phase II reactions
Phase I reactions are ‘Preparatory Reactions’ as they are non-synthetic modification reactions and include oxidation, reduction, hydrolysis, hydroxylation, or demethylation. They are facilitated by the cytochrome P (CYP) 450 family and convert a parent drug to a polar metabolite by introducing or unmasking a more polar site. Phase I enzymes, although present since birth, vary in their levels and activity and reach maturity by the first year of life (Table 1).
Table 1
| CYP isoforms | Postnatal age of expression | Drugs | Inducers |
|---|---|---|---|
| CYP 2E1 | Hours | Ethanol paracetamol | Ethanol, isoniazid |
| CYP 2D6 | Hours, days | Amphetamines, codeine lignocaine, metoclopramide | Phenobarbital, phenytoin |
| CYP 2C9 | First weeks | Ibuprofen, phenytoin | Rifampicin |
| CYP 2C19 | First weeks | Omeprazole, phenytoin diazepam indomethacin | Carbamazepine, prednisone |
| CYP 3A4 | First weeks | Steroids, clarithromycin | Phenobarbitone, phenytoin |
| CYP 1A2 | 1–3 months | Caffeine, acetaminophen | Insulin, omeprazole |
CYP, cytochrome.
Phase II reactions are synthetic reactions mediated by enzymes such as UDP-glucuronosyltransferase, glutathione S-transferase, N-acetyltransferase, or sulfotransferase (63,64,69). These add endogenous substances to the drug to form a highly polar metabolite by glucuronidation, sulfation, acetylation, or methylation reactions. Expression of phase II enzymes varies with maturation and development. Sulfation is relatively developed at birth compared to acetylation and glucuronidation, which develop with postnatal age and weight gain. As morphine and acetaminophen involve glucuronidation for their metabolism, their clearance is low in neonates (70,71). Acetaminophen compensates glucuronidation deficiencies at birth by predominant sulfation and is excreted as sulfate-conjugated metabolites.
Hepatic CYP P450 mediated metabolism
Hepatic CYP is a group of microsomal enzymes localized in the smooth endoplasmic reticulum. They are primarily responsible for phase I oxidative metabolism (72). More than 1,000 CYP450s are known, but only about 50 are functionally active in humans. CYP1, CYP2, and CYP3 families are involved in most drug metabolism reactions. The reactions catalyzed by them include aromatic ring and aliphatic side chain hydroxylation, dealkylation, deamination, dehalogenation, sulfoxidation, N-oxidation, N-hydroxylation, nitro reduction, and azo reduction. Although individual CYP isoforms tend to have substrate specificities, but overlap is common (73).
During human development, CYP isoforms are expressed differentially and are categorized into three groups. First group enzymes have the highest levels in fetal life, their activities reduce with increasing age, and include isoforms such as CYP3A7, SULT1A3/1A4. The second group includes enzymes that are expressed after birth and increase progressively with age. They include CYP3A5, CYP2C19, and SULT1A1. Enzymes such as CYP2D6, CYP3A4, CYP2C9, and CYP1A2 are assigned to the third group, these enzymes display modest ontogeny during the second or third trimester of pregnancy, with increased phenotypic expression throughout infancy (10,12,15,70,73-76).
CYP3A4 and 3A5 are responsible for 50% of drug metabolism. CYP3A4 increases concomitantly in the first year of life and becomes the major isoform in the adult liver. CYP2C and CYP2D6 activity develop in fetal life and increase during the perinatal period, however, it is still very low compared to that in an adult liver. The cytochrome P450 genes also exhibit single nucleotide polymorphisms that lead to wide genetic variation which further explains the differential enzymatic activity. For example, CYP2D6 metabolizes codeine to morphine; consequently, neonates with higher CYP2D6 activity may influence morphine toxicity (73,77,78).
Drugs such as fluconazole, spironolactone, and metronidazole can inhibit CYP450 enzymatic activity. This inhibition reduces the metabolism of potential substrates and secondarily delays their elimination. Drugs such as phenobarbitone and rifampicin can induce CYP3A, and CYP2C respectively, which may prolong the half-life of certain drugs. Common cytochrome isoforms their variable age of expression, substrates, and inducers are represented in Table 1.
Extra microsomal enzymes in the mitochondria and cytosol of the liver and other tissues may also mediate some important phase I reactions. These include aldehyde dehydrogenases, tyrosine hydroxylase, and monoamine oxidase. Their expression is also low in the early postnatal period but improves with postnatal age (73,79,80).
Drug elimination
Elimination refers to the process of clearance of a drug from the body. Kidney and hepatobiliary systems are the main routes of clearance of drugs and their metabolites. The primary mode of excretion is through the kidney while the liver is the main site of metabolic clearance. Exhaled air, saliva, and sweat also contribute to the elimination of certain drugs.
The site of elimination
As mentioned earlier, the kidneys and liver are the primary sites of drug elimination.
Drug elimination by kidney
The kidney plays a major role in the elimination of drugs. The excretion of drugs through the kidney is determined by interrelated factors such as renal blood flow, glomerular filtration rate (GFR), and renal tubular activity (reabsorption and secretion). These are not adequately developed in premature infants. Renal function improves with gestational/postnatal age and maturation of the neonate, which is accompanied by changes in renal blood flow and renal vascular resistance (81). Even though GFR has a linear relationship with gestational age, this holds only after 34 weeks of gestation. In term neonates, GFR doubles within 2 weeks of life and attains adult value by two and a half months of life. However, it can take 2 years for a preterm infant to attain the same level. This extensive variability is explained by incomplete kidney maturation during infancy. Drugs such as digoxin, aminoglycosides, and cephalosporins are dependent on GFR for excretion, and changes in the GFR can lead to potential toxicity. On the other hand, drugs depending on tubular secretion for elimination have decreased clearance such as furosemide and penicillins (12,65). Small for gestational age (SGA) infants exhibit decreased clearance of vancomycin and amikacin up to 16% when compared to appropriate for gestational age infants (82).
Prenatal exposure to drugs also modifies the excretion capacities of the kidneys. For example, methadone increases GFR, whereas indomethacin reduces it. Alterations in plasma and urine pH can also alter the excretion of ionized drugs, especially weak acids, and bases (73).
Consequently, factors such as maturation (e.g., age, birth weight), disease characteristics (e.g., peripartum asphyxia, renal congenital malformations, co-medication, or growth restriction), genetic polymorphisms, and renal failure may adversely impact the excretion of many drugs. Creatinine clearance and nomograms are used to modify drug dosage in infants with renal failure. However, they should be used with caution as they are primarily designed for adults and children. Hence, there is a compelling need to develop gestational-specific nomograms based on gestational age and postnatal age (10).
The excretion of excipients, which are often coadministered with drugs, is often neglected in neonatal population. They can be administered in significant amounts in neonates and have been associated with significant adverse effects including mortality. Classic examples of these include propylene glycol and benzyl alcohol (77,83-88).
Drug elimination in liver
The liver is an important organ for metabolic drug elimination. Internal clearance is absent at birth but develops later. Phase I and phase II reactions play a significant role in drug metabolism and facilitate elimination as elucidated earlier. Postnatal maturation leads to differences in phase I and phase II reactions. These qualitative and quantitative differences exist because of different stages of development and influence the rate and pattern of elimination by newborns (62-65). Other factors that play an important role in hepatic clearance are blood flow and plasma protein binding.
Drugs eliminated by liver are classified further as ‘flow-limited’ or ‘capacity-limited’. A drug that relies on hepatic blood flow for clearance is classified as ‘flow-limited’. A change in hepatic blood flow has a proportional change on the delivery of the drug to the liver and hence, on its overall clearance. Examples of this includes drugs like morphine, propranolol, and meperidine. If a drug depends on hepatic metabolizing capacity for clearance, it is classified as ‘capacity limited’. Hepatic metabolism determines the clearance, which is independent of hepatic blood flow (2,89). Examples of this includes drugs like phenytoin, theophylline, and diazepam (2,48,89).
The cardiovascular status also impacts drug excretion. Congestive cardiac failure and cardiac defects alter regional blood flow distribution. It may impact renal and hepatic blood flow as well as function and consequently diminishing drug biotransformation and elimination.
The compartment of metabolism
The distribution of drugs in different tissues of the body has led to the ‘compartmental modeling’ concept. Central compartments include highly perfused tissues such as the heart, liver, lungs, and kidneys, whereas the peripheral compartments constitute tissues such as fat, muscle, and cerebrospinal fluid (CSF). Although drugs are not restricted to one compartment, the ‘one-compartment’ model is most frequently cited. This holds for drugs with insignificant distribution in the peripheral/extravascular compartments, such as aminoglycosides. It is also based on the assumption that a drug distributes instantly to all body tissues and fluids. The distribution phase (alpha-phase) is very short (15 to 20 min) but the metabolism and elimination phases (beta-phase or terminal phase) are much slower and longer (48,90).
On the other hand, some drugs follow a complex ‘multicompartmental model’. This includes lipophilic drugs such as benzodiazepines or those with extensive tissue or intracellular uptake. Plasma drug samples should be withdrawn only after the distribution of a drug is complete. This permits a reflection of accurate drug levels as a state of pseudo equilibrium is reached between the peripheral and central compartments (15,90).
Clearance (Cl)
Cl of a drug refers to the theoretical volume of plasma from which the drug is completely removed in a unit of time and is expressed as volume over time. It can be calculated as
where C is the plasma concentration of the drug.
Cl represents the volume of plasma from which the drug is completely removed in a given time. Cl determines the steady-state concentration for a given dose (49). Based on the rate of clearance, drugs are said to follow first-order or zero-order kinetics. In first-order kinetics, a fraction of drug is excreted per unit time, clearance remains constant. There is increase in elimination of the drug with increased amount in the body. Drug follows a linear plot between the plasma concentration versus time. In contrast, zero-order kinetics of elimination is characterized by a constant rate of elimination, irrespective of drug concentration in the body. It is also known as ‘saturation kinetics’ or Michaelis-Menten kinetics. A constant amount of the drug is eliminated over time, regardless of the amount of drug in the body. The Cl decreases with increases in concentration. Drugs with zero-order kinetics include caffeine, chloramphenicol, diazepam, furosemide, indomethacin, and phenytoin (15,91-95).
Most of the drugs used in neonatology display first-order kinetics, but some drugs transition to zero-order kinetics at higher doses. As a result, plasma concentration increases disproportionately with increases in dose, which can lead to toxicity and adverse reactions. This is classically seen with phenytoin and theophylline metabolism (15,91-95).
Half-life
Half-life (t1⁄2) is the time required for the serum concentration of a drug to decrease by 50% after absorption and elimination are complete. It determines the time to reach a steady state which is usually three to five half-lives and to ascertain appropriate drug dosing intervals. Half-life depends on Vd and Cl. Drug half-life determines the speed at which drug concentration decreases in serum. Nearly complete drug elimination occurs in 4–5 half-lives. For drugs eliminated by first-order kinetics, t½ remains constant as Vd and Cl do not change with dose. Examples of this includes most drugs used in neonates. Whereas drugs eliminated by zero-order kinetics, t1/2 increases with dose because Cl progressively decreases as the dose is increased. Examples of this includes drugs such as caffeine, chloramphenicol, diazepam, furosemide, indomethacin, and phenytoin (48,94,95).
Steady-state plasma concentration
When a drug is repeatedly administered at relatively short intervals, it accumulates in the body till a state of balance is achieved between intake and excretion. This balanced state is called a steady-state plasma concentration (Cpss). It is important to maintain steady drug levels, so that the efficacy of a drug remains constant, provided there is no tolerance to the drug. Steady-state is reached in 4–5 half-lives. Smaller doses repeated more frequently help maintain Cpss. If repeated at too short of intervals, it can cause potential side effects. A compromise between loss of efficacy at troughs and side effects at peaks determines dose intervals. The average Cpss is approximately 1/3 of the minimal and maximal levels with enteral drugs, as absorption takes longer. This holds true for drugs that follow first-order kinetics and have a linear curve. There is increase in Cpss, which is out of proportion to dose change, when it increases beyond saturation levels for drugs following zero-order kinetics (95).
Loading dose and maintenance dose
It is well known that it takes about four t1/2 to attain a steady state, which might delay the therapeutic efficacy of some drugs. Loading doses are used for such drugs to attain faster peak effective concentration for optimal therapeutic effect. The subsequent maintenance doses are used to keep the drug plasma concentration at optimum levels. In neonates, drugs such as aminophylline, caffeine, and anticonvulsants are used with these principles (96). The maintenance dose is determined by Cl and t1/2. It is useful for drugs with a short half-life and in critically ill neonates to achieve and maintain effective drugs concentration.
Therapeutic drug monitoring in neonates
In neonates, therapeutic drug monitoring refers to the measurement of plasma drug concentration. This provides an estimate of the pharmacokinetic variables in a particular patient and estimates the magnitude of deviation from an average patient. It is helpful to make appropriate adjustments in the dosage regimen. The clinical benefits can be maximized without added toxic or adverse reactions. TDM is particularly useful in renal failure, and with drugs that have a low safety margin or are potentially toxic, such as theophylline, aminoglycosides, and vancomycin. TDM is also useful when there is a failure of response without any apparent reason (i.e., antimicrobials). Accurate determination of TDM depends on appropriate intervals between drug administration and drawing of blood, the nature of the drug, and even on the purpose of TDM. It should then be repeated at specific intervals to monitor the progress. Immature renal function in preterm makes them susceptible to overdosing. This assumes even more significance for drugs such as glycopeptides and aminoglycosides, which are eliminated exclusively by the renal route (97). Additionally, betamethasone and indomethacin, when administered to the fetus, alter renal maturation after birth (95,98).
TDM a tool for titrating drugs
TDM is a standard tool for analyzing many drug concentrations given inter-individual variability in pharmacokinetics and to avoid toxic and substance susceptibility such as ototoxicity and nephrotoxicity (99). For gentamicin, an aminoglycoside, TDM is a standard tool to determine target peak and trough concentrations. Some anti-fungal agents such as itraconazole and voriconazole have a narrow therapeutic index, given the wide differences in volume distribution, clearance, and other pharmacokinetic parameters, especially in the neonatal period (100-102). With genetic polymorphism of CYP2C19 monitoring of drug concentration via TDM is ideal (100,103). Phenobarbital and phenytoin are amongst the most commonly prescribed antiepileptic drugs in newborns, although there are concerns about their potential neurotoxicity on the developing brain. They are mostly administered starting with a loading dose, followed by maintenance doses and TDM is extensively used for their monitoring (1,104-107).
Pharmacokinetics of drugs during therapeutic hypothermia (TH)
In term newborns with moderate to severe perinatal asphyxia, TH is now a standard of care. However, pharmacokinetics changes have been described during that period, such as the effect on decreased drug clearance by glomerular filtration and decreased activity of hepatic cytochrome enzymes due to the effect on blood flow and cardiac output (108,109). Consequently, anti-epileptics, such as phenobarbital, when administered during TH for neonatal seizures resulted in higher concentration in plasma and longer half-lives than expected compared to normothermic newborns (110). However, in a Dutch study no clinically significant effect of TH on phenobarbital was observed (111). A ParmaCool study advised a Phenobarbital dose of 30 mg/kg (instead of 20 mg/kg) to reach therapeutic concentrations during TH (112). Certain aminoglycosides such as amikacin and gentamicin exhibit concern with a decrease in clearance during TH. A 12-h increase in dosing interval was suggested for reduced clearance (113). A meta-analysis reviewed eight studies and confirmed this decreased clearance of gentamicin during TH. They proposed modified gentamicin dosing regimens with 36-h intervals (114). Furthermore, Cies et al. suggested modified dosing of ampicillin to 25–50 mg/kg/day for antimicrobial action in the setting of controlled hypothermia (115).
Conclusions
With increasing survival rates of extremely premature babies, understanding pharmacokinetics and its principles are vital for optimizing treatment in the unique neonatal population. As opposed to ‘adult’ drug pharmacokinetics principles, it is necessary to understand complex drug interactions and to achieve therapeutically adequate drug concentrations, with the lowest risk of hazards possible. Neonatal physiology, disease states, developmental and maturational differences have a profound impact on drug pharmacokinetics and these diversities must be kept in mind while administering medications. Vast inter- and intra-patient differences in drug handling make it indispensable for safe and effective drug administration. TDM is a vital tool and must be utilized to individualize the dosing of drugs in this vulnerable population whenever feasible. It must be acknowledged that not many drugs have their TDM range determined, underlying a potential area of research to optimize treatment with these drugs. While the number of drugs whose TDM is available is small, it is important to use it wherever available for optimal neonatal dosing. The success of treatment is determined by complex interactions between the administered drug, the host, and the disease process. Clinical research must be encouraged in premature and term neonates at different postnatal ages to establish pharmacokinetic models specific to neonates for effective dosing regimens and avoidance of toxic levels.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-22-11/coif). K.Y. serves as an unpaid editorial board member of Pediatric Medicine from December 2022 to November 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Rose DU, Cairoli S, Dionisi M, et al. Therapeutic Drug Monitoring Is a Feasible Tool to Personalize Drug Administration in Neonates Using New Techniques: An Overview on the Pharmacokinetics and Pharmacodynamics in Neonatal Age. Int J Mol Sci 2020;21:5898. [Crossref] [PubMed]
- Nies AS, Shand DG, Wilkinson GR. Altered hepatic blood flow and drug disposition. Clin Pharmacokinet 1976;1:135-55. [Crossref] [PubMed]
- Allegaert K, Verbesselt R, Naulaers G, et al. Developmental pharmacology: neonates are not just small adults. Acta Clin Belg 2008;63:16-24. [Crossref] [PubMed]
- Allegaert K, van den Anker JN. Clinical pharmacology in neonates: small size, huge variability. Neonatology 2014;105:344-9. [Crossref] [PubMed]
- van den Anker J, Reed MD, Allegaert K, et al. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J Clin Pharmacol 2018;58:S10-25. [Crossref] [PubMed]
- Shinwell ES, Eventov-Friedman S. Impact of perinatal corticosteroids on neuromotor development and outcome: review of the literature and new meta-analysis. Semin Fetal Neonatal Med 2009;14:164-70. [Crossref] [PubMed]
- Zaffanello M, Bassareo PP, Cataldi L, et al. Long-term effects of neonatal drugs on the kidney. J Matern Fetal Neonatal Med 2010;23:87-9. [Crossref] [PubMed]
- Smits A, Kulo A, van den Anker J, et al. The amikacin research program: a stepwise approach to validate dosing regimens in neonates. Expert Opin Drug Metab Toxicol 2017;13:157-66. [Crossref] [PubMed]
- Allegaert K, Simons SHP, Tibboel D, et al. Non-maturational covariates for dynamic systems pharmacology models in neonates, infants, and children: Filling the gaps beyond developmental pharmacology. Eur J Pharm Sci 2017;109S:S27-31. [Crossref] [PubMed]
- Allegaert K, van de Velde M, van den Anker J. Neonatal clinical pharmacology. Paediatr Anaesth 2014;24:30-8. [Crossref] [PubMed]
- Mian P, Flint RB, Tibboel D, et al. Therapeutic Drug Monitoring in Neonates: What Makes them Unique? Curr Pharm Des 2017;23:5790-800. [Crossref] [PubMed]
- Allegaert K, Mian P, van den Anker JN. Developmental Pharmacokinetics in Neonates: Maturational Changes and Beyond. Curr Pharm Des 2017;23:5769-78. [Crossref] [PubMed]
- Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med 2003;349:1157-67. [Crossref] [PubMed]
- Roberts JA, Kumar A, Lipman J. Right Dose, Right Now: Customized Drug Dosing in the Critically Ill. Crit Care Med 2017;45:331-6. [Crossref] [PubMed]
- Johnson PJ. Neonatal pharmacology--pharmacokinetics. Neonatal Netw 2011;30:54-61. [Crossref] [PubMed]
- Linakis MW, Roberts JK, Lala AC, et al. Challenges Associated with Route of Administration in Neonatal Drug Delivery. Clin Pharmacokinet 2016;55:185-96. [Crossref] [PubMed]
- Avery GB, Randolph JG, Weaver T. Gastric acidity in the first day of life. Pediatrics 1966;37:1005-7. [Crossref] [PubMed]
- Ebers DW, Gibbs GE, Smith DI. Gastric acidity on the first day of life. Pediatrics 1956;18:800-2. [Crossref] [PubMed]
- Agunod M, Yamaguchi N, Lopez R, et al. Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. Am J Dig Dis 1969;14:400-14. [Crossref] [PubMed]
- Deren JS. Development of structure and function in the fetal and newborn stomach. Am J Clin Nutr 1971;24:144-59. [Crossref] [PubMed]
- Kelly EJ, Newell SJ, Brownlee KG, et al. Gastric acid secretion in preterm infants. Early Hum Dev 1993;35:215-20. [Crossref] [PubMed]
- Huang NN, High RH. Comparison of serum levels following the administration of oral and parenteral preparations of penicillin to infants and children of various age groups. J Pediatr 1953;42:657-8. [Crossref] [PubMed]
- Silverio J, Poole JW. Serum concentrations of ampicillin in newborn infants after oral administration. Pediatrics 1973;51:578-80. [Crossref] [PubMed]
- Morselli PL, Franco-Morselli R, Bossi L. Clinical pharmacokinetics in newborns and infants. Age-related differences and therapeutic implications. Clin Pharmacokinet 1980;5:485-527. [Crossref] [PubMed]
- Wallin A, Jalling B, Boréus LO. Plasma concentrations of phenobarbital in the neonate during prophylaxis for neonatal hyperbilirubinemia. J Pediatr 1974;85:392-7. [Crossref] [PubMed]
- Lebenthal E, Lee PC. Development of functional responses in human exocrine pancreas. Pediatrics 1980;66:556-60. [Crossref] [PubMed]
- Barbara L, Lazzari R, Roda A, et al. Serum bile acids in newborns and children. Pediatr Res 1980;14:1222-5. [Crossref] [PubMed]
- Murphy GM, Signer E. Bile acid metabolism in infants and children. Gut 1974;15:151-63. [Crossref] [PubMed]
- Boehm G, Borte M, Müller H, et al. Activities of trypsin and lipase in duodenal aspirates of preterm infants: influence of dietary protein and fat composition. Am J Clin Nutr 1995;61:524-7. [Crossref] [PubMed]
- Berseth CL. Gestational evolution of small intestine motility in preterm and term infants. J Pediatr 1989;115:646-51. [Crossref] [PubMed]
- Gupta M, Brans YW. Gastric retention in neonates. Pediatrics 1978;62:26-9. [Crossref] [PubMed]
- Berseth CL. Effect of early feeding on maturation of the preterm infant's small intestine. J Pediatr 1992;120:947-53. [Crossref] [PubMed]
- Cavell B. Gastric emptying in infants fed human milk or infant formula. Acta Paediatr Scand 1981;70:639-41. [Crossref] [PubMed]
- Meyer R, Foong RX, Thapar N, et al. Systematic review of the impact of feed protein type and degree of hydrolysis on gastric emptying in children. BMC Gastroenterol 2015;15:137. [Crossref] [PubMed]
- Tsuji A, Tamai I. Carrier-mediated intestinal transport of drugs. Pharm Res 1996;13:963-77. [Crossref] [PubMed]
- Benet LZ, Cummins CL. The drug efflux-metabolism alliance: biochemical aspects. Adv Drug Deliv Rev 2001;50:S3-11. [Crossref] [PubMed]
- Mooij MG, de Koning BE, Lindenbergh-Kortleve DJ, et al. Human Intestinal PEPT1 Transporter Expression and Localization in Preterm and Term Infants. Drug Metab Dispos 2016;44:1014-9. [Crossref] [PubMed]
- Mooij MG, Schwarz UI, de Koning BA, et al. Ontogeny of human hepatic and intestinal transporter gene expression during childhood: age matters. Drug Metab Dispos 2014;42:1268-74. [Crossref] [PubMed]
- Severijnen R, Bayat N, Bakker H, et al. Enteral drug absorption in patients with short small bowel: a review. Clin Pharmacokinet 2004;43:951-62. [Crossref] [PubMed]
- de Wildt SN, Kearns GL, Hop WC, et al. Pharmacokinetics and metabolism of oral midazolam in preterm infants. Br J Clin Pharmacol 2002;53:390-2. [Crossref] [PubMed]
- Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev 2003;55:667-86. [Crossref] [PubMed]
- Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology 1984;86:174-93. [Crossref] [PubMed]
- Pokorná P, Šíma M, Černá O, et al. Actual body weight-based vancomycin dosing in neonates. J Chemother 2019;31:307-12. [Crossref] [PubMed]
- Huang CT, Rodriguez JT, Woodward WE, et al. Comparison of patterns of fecal bile acid and neutral sterol between children and adults. Am J Clin Nutr 1976;29:1196-203. [Crossref] [PubMed]
- Sheng KT, Huang NN, Promadhattavedi V. Serum concentrations of cephalothin in infants and children and placental transmission of the antibiotic. Antimicrob Agents Chemother (Bethesda) 1964;10:200-6. [PubMed]
- Germovsek E, Barker CI, Sharland M. What do I need to know about aminoglycoside antibiotics? Arch Dis Child Educ Pract Ed 2017;102:89-93. [Crossref] [PubMed]
- West DP, Worobec S, Solomon LM. Pharmacology and toxicology of infant skin. J Invest Dermatol 1981;76:147-50. [Crossref] [PubMed]
- Reiter PD. Neonatal Pharmacology and Pharmacokinetics. Neoreviews 2002;3:e229-36. [Crossref]
- van Hoogdalem E, de Boer AG, Breimer DD. Pharmacokinetics of rectal drug administration, Part I. General considerations and clinical applications of centrally acting drugs. Clin Pharmacokinet 1991;21:11-26. [Crossref] [PubMed]
- Greenough A, Papalexopoulou N. The roles of drug therapy given via the endotracheal tube to neonates. Arch Dis Child Fetal Neonatal Ed 2017;102:F277-81. [Crossref] [PubMed]
- Heimann G. Enteral absorption and bioavailability in children in relation to age. Eur J Clin Pharmacol 1980;18:43-50. [Crossref] [PubMed]
- FRIIS-HANSEN B. Body water compartments in children: changes during growth and related changes in body composition. Pediatrics 1961;28:169-81. [Crossref] [PubMed]
- Friis-Hansen B. Body composition during growth. In vivo measurements and biochemical data correlated to differential anatomical growth. Pediatrics 1971;47:264. [PubMed]
- Assael BM. Pharmacokinetics and drug distribution during postnatal development. Pharmacol Ther 1982;18:159-97. [Crossref] [PubMed]
- van den Anker JN, Coppes MJ, Koren G. Neonatal and pediatric clinical pharmacology. Pediatr Clin North Am 2012;59:xv-xviii. [Crossref] [PubMed]
- Fonzo-Christe C, Guignard B, Zaugg C, et al. Impact of clinical decision support guidelines on therapeutic drug monitoring of gentamicin in newborns. Ther Drug Monit 2014;36:656-62. [Crossref] [PubMed]
- Ehrnebo M, Agurell S, Jalling B, et al. Age differences in drug binding by plasma proteins: studies on human foetuses, neonates and adults. Eur J Clin Pharmacol 1971;3:189-93. [Crossref] [PubMed]
- Herngren L, Ehrnebo M, Boréus LO. Drug binding to plasma proteins during human pregnancy and in the perinatal period. Studies on cloxacillin and alprenolol. Dev Pharmacol Ther 1983;6:110-24. [Crossref] [PubMed]
- Rane A, Lunde PK, Jalling B, et al. Plasma protein binding of diphenylhydantoin in normal and hyperbilirubinemic infants. J Pediatr 1971;78:877-82. [Crossref] [PubMed]
- Besunder JB, Reed MD, Blumer JL. Principles of drug biodisposition in the neonate. A critical evaluation of the pharmacokinetic-pharmacodynamic interface (Part I). Clin Pharmacokinet 1988;14:189-216. [Crossref] [PubMed]
- Pacifici GM. Pharmacokinetics of cephalosporins in the neonate: a review. Clinics (Sao Paulo) 2011;66:1267-74. [Crossref] [PubMed]
- Allegaert K, Rayyan M, Vanhaesebrouck S, et al. Developmental pharmacokinetics in neonates. Expert Rev Clin Pharmacol 2008;1:415-28. [Crossref] [PubMed]
- Blake MJ, Castro L, Leeder JS, et al. Ontogeny of drug metabolizing enzymes in the neonate. Semin Fetal Neonatal Med 2005;10:123-38. [Crossref] [PubMed]
- Hines RN. Developmental expression of drug metabolizing enzymes: impact on disposition in neonates and young children. Int J Pharm 2013;452:3-7. [Crossref] [PubMed]
- Smits A, Kulo A, de Hoon JN, et al. Pharmacokinetics of drugs in neonates: pattern recognition beyond compound specific observations. Curr Pharm Des 2012;18:3119-46. [Crossref] [PubMed]
- Pacifici GM. Clinical Pharmacology of Phenobarbital in Neonates: Effects, Metabolism and Pharmacokinetics. Curr Pediatr Rev 2016;12:48-54. [Crossref] [PubMed]
- Šíma M, Michaličková D, Slanař O. What is the Best Predictor of Phenobarbital Pharmacokinetics to Use for Initial Dosing in Neonates? Pharmaceutics 2021;13:301. [Crossref] [PubMed]
- Yukawa M, Yukawa E, Suematsu F, et al. Population pharmacokinetics of phenobarbital by mixed effect modelling using routine clinical pharmacokinetic data in Japanese neonates and infants: an update. J Clin Pharm Ther 2011;36:704-10. [Crossref] [PubMed]
- van Groen BD, Nicolaï J, Kuik AC, et al. Ontogeny of Hepatic Transporters and Drug-Metabolizing Enzymes in Humans and in Nonclinical Species. Pharmacol Rev 2021;73:597-678. [Crossref] [PubMed]
- Knibbe CA, Krekels EH, van den Anker JN, et al. Morphine glucuronidation in preterm neonates, infants and children younger than 3 years. Clin Pharmacokinet 2009;48:371-85. [Crossref] [PubMed]
- Flint RB, Roofthooft DW, van Rongen A, et al. Exposure to acetaminophen and all its metabolites upon 10, 15, and 20 mg/kg intravenous acetaminophen in very-preterm infants. Pediatr Res 2017;82:678-84. [Crossref] [PubMed]
- Guengerich FP, Hosea NA, Parikh A, et al. Twenty years of biochemistry of human P450s: purification, expression, mechanism, and relevance to drugs. Drug Metab Dispos 1998;26:1175-8. [PubMed]
- Polin RA, Rowitch DH, Abman SH, et al. editors. Fetal and neonatal physiology. 6th ed. Philadelphia, Pennsylvania: Elsevier; 2021.
- van den Anker JN. Developmental pharmacology. Dev Disabil Res Rev 2010;16:233-8. [Crossref] [PubMed]
- de Wildt SN. Profound changes in drug metabolism enzymes and possible effects on drug therapy in neonates and children. Expert Opin Drug Metab Toxicol 2011;7:935-48. [Crossref] [PubMed]
- Shimada T, Yamazaki H, Mimura M, et al. Characterization of microsomal cytochrome P450 enzymes involved in the oxidation of xenobiotic chemicals in human fetal liver and adult lungs. Drug Metab Dispos 1996;24:515-22. [PubMed]
- O'Hara K, Wright IM, Schneider JJ, et al. Pharmacokinetics in neonatal prescribing: evidence base, paradigms and the future. Br J Clin Pharmacol 2015;80:1281-8. [Crossref] [PubMed]
- Van Donge T, Mian P, Tibboel D, et al. Drug metabolism in early infancy: opioids as an illustration. Expert Opin Drug Metab Toxicol 2018;14:287-301. [Crossref] [PubMed]
- Hines RN, McCarver DG. The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J Pharmacol Exp Ther 2002;300:355-60. [Crossref] [PubMed]
- Mørk ML, Andersen JT, Lausten-Thomsen U, et al. The Blind Spot of Pharmacology: A Scoping Review of Drug Metabolism in Prematurely Born Children. Front Pharmacol 2022;13:828010. [Crossref] [PubMed]
- van den Anker JN, Schoemaker RC, Hop WC, et al. Ceftazidime pharmacokinetics in preterm infants: effects of renal function and gestational age. Clin Pharmacol Ther 1995;58:650-9. [Crossref] [PubMed]
- Allegaert K, Anderson BJ, van den Anker JN, et al. Renal drug clearance in preterm neonates: relation to prenatal growth. Ther Drug Monit 2007;29:284-91. [Crossref] [PubMed]
- Lass J, Naelapää K, Shah U, et al. Hospitalised neonates in Estonia commonly receive potentially harmful excipients. BMC Pediatr 2012;12:136. [Crossref] [PubMed]
- Shehab N, Lewis CL, Streetman DD, et al. Exposure to the pharmaceutical excipients benzyl alcohol and propylene glycol among critically ill neonates. Pediatr Crit Care Med 2009;10:256-9. [Crossref] [PubMed]
- Souza A Jr, Santos D, Fonseca S, et al. Toxic excipients in medications for neonates in Brazil. Eur J Pediatr 2014;173:935-45. [Crossref] [PubMed]
- Kulo A, de Hoon JN, Allegaert K. The propylene glycol research project to illustrate the feasibility and difficulties to study toxicokinetics in neonates. Int J Pharm 2012;435:112-4. [Crossref] [PubMed]
- De Cock RF, Knibbe CA, Kulo A, et al. Developmental pharmacokinetics of propylene glycol in preterm and term neonates. Br J Clin Pharmacol 2013;75:162-71. [Crossref] [PubMed]
- Robertson AF. Reflections on errors in neonatology III. The "experienced" years, 1970 to 2000. J Perinatol 2003;23:240-9. [Crossref] [PubMed]
- Wilkinson GR, Shand DG. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther 1975;18:377-90. [Crossref] [PubMed]
- Ahmed TA. Pharmacokinetics of Drugs Following IV Bolus, IV Infusion, and Oral Administration. InTech; 2015.
- Pacifici GM. Clinical pharmacology of theophylline in preterm infants: effects, metabolism and pharmacokinetics. Curr Pediatr Rev 2014;10:297-303. [Crossref] [PubMed]
- Bourgeois BF, Dodson WE. Phenytoin elimination in newborns. Neurology 1983;33:173-8. [Crossref] [PubMed]
- Veroniki AA, Rios P, Cogo E, et al. Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: a systematic review and network meta-analysis. BMJ Open 2017;7:e017248. [Crossref] [PubMed]
- Katzung BG, Todd WV. Basic and clinical pharmacology. 15th ed. New York: McGraw-Hill Education; 2021.
- Tripathi KD. Essentials of Medical Pharmacology. New Delhi: Jaypee Brothers Medical Publishers; 2004.
- Tetelbaum M, Finkelstein Y, Nava-Ocampo AA, et al. Back to basics: understanding drugs in children: pharmacokinetic maturation. Pediatr Rev 2005;26:321-8. [Crossref] [PubMed]
- Abdulla A, Edwina EE, Flint RB, et al. Model-Informed Precision Dosing of Antibiotics in Pediatric Patients: A Narrative Review. Front Pediatr 2021;9:624639. [Crossref] [PubMed]
- van den Anker JN, Hop WC, de Groot R, et al. Effects of prenatal exposure to betamethasone and indomethacin on the glomerular filtration rate in the preterm infant. Pediatr Res 1994;36:578-81. [Crossref] [PubMed]
- Pacifici GM. Clinical pharmacokinetics of aminoglycosides in the neonate: a review. Eur J Clin Pharmacol 2009;65:419-27. [Crossref] [PubMed]
- John J, Loo A, Mazur S, et al. Therapeutic drug monitoring of systemic antifungal agents: a pragmatic approach for adult and pediatric patients. Expert Opin Drug Metab Toxicol 2019;15:881-95. [Crossref] [PubMed]
- Zheng YZ, Wang S. Advances in antifungal drug measurement by liquid chromatography-mass spectrometry. Clin Chim Acta 2019;491:132-45. [Crossref] [PubMed]
- Scott BL, Hornik CD, Zimmerman K. Pharmacokinetic, efficacy, and safety considerations for the use of antifungal drugs in the neonatal population. Expert Opin Drug Metab Toxicol 2020;16:605-16. [Crossref] [PubMed]
- McCreary EK, Bayless M, Van AP, et al. Impact of Triazole Therapeutic Drug Monitoring Availability and Timing. Antimicrob Agents Chemother 2019;63:e01245-19. [Crossref] [PubMed]
- Dilena R, De Liso P, Di Capua M, et al. Influence of etiology on treatment choices for neonatal seizures: A survey among pediatric neurologists. Brain Dev 2019;41:595-9. [Crossref] [PubMed]
- Donovan MD, Griffin BT, Kharoshankaya L, et al. Pharmacotherapy for Neonatal Seizures: Current Knowledge and Future Perspectives. Drugs 2016;76:647-61. [Crossref] [PubMed]
- Pauwels S, Allegaert K. Therapeutic drug monitoring in neonates. Arch Dis Child 2016;101:377-81. [Crossref] [PubMed]
- Touw DJ, van den Anker JN. Therapeutic Drug Monitoring of Antimicrobial Drugs in Neonates: An Opinion Article. Ther Drug Monit 2022;44:65-74. [Crossref] [PubMed]
- Wildschut ED, van Saet A, Pokorna P, et al. The impact of extracorporeal life support and hypothermia on drug disposition in critically ill infants and children. Pediatr Clin North Am 2012;59:1183-204. [Crossref] [PubMed]
- El-Dib M, Soul JS. The use of phenobarbital and other anti-seizure drugs in newborns. Semin Fetal Neonatal Med 2017;22:321-7. [Crossref] [PubMed]
- Filippi L, la Marca G, Cavallaro G, et al. Phenobarbital for neonatal seizures in hypoxic ischemic encephalopathy: a pharmacokinetic study during whole body hypothermia. Epilepsia 2011;52:794-801. [Crossref] [PubMed]
- van den Broek MP, Groenendaal F, Toet MC, et al. Pharmacokinetics and clinical efficacy of phenobarbital in asphyxiated newborns treated with hypothermia: a thermopharmacological approach. Clin Pharmacokinet 2012;51:671-9. [Crossref] [PubMed]
- Hutchinson L, Sinclair M, Reid B, et al. A descriptive systematic review of salivary therapeutic drug monitoring in neonates and infants. Br J Clin Pharmacol 2018;84:1089-108. [Crossref] [PubMed]
- Cristea S, Smits A, Kulo A, et al. Amikacin Pharmacokinetics To Optimize Dosing in Neonates with Perinatal Asphyxia Treated with Hypothermia. Antimicrob Agents Chemother 2017;61:e01282-17. [Crossref] [PubMed]
- Choi DW, Park JH, Lee SY, et al. Effect of hypothermia treatment on gentamicin pharmacokinetics in neonates with hypoxic-ischaemic encephalopathy: A systematic review and meta-analysis. J Clin Pharm Ther 2018;43:484-92. [Crossref] [PubMed]
- Cies JJ, Fugarolas KN, Moore WS 2nd, et al. Population Pharmacokinetics and Pharmacodynamic Target Attainment of Ampicillin in Neonates with Hypoxemic-Ischemic Encephalopathy in the Setting of Controlled Hypothermia. Pharmacotherapy 2017;37:456-63. [Crossref] [PubMed]
Cite this article as: Bansal N, Momin S, Bansal R, Gurram Venkata SKR, Ruser L, Yusuf K. Pharmacokinetics of drugs: newborn perspective. Pediatr Med 2024;7:19.