
Ischemic stroke associated with Multisystemic Inflammatory Syndrome in children after SARS-CoV-2 infection: a case report
Highlight box
Key findings
• Analyze ischemic cerebrovascular disease in the context of multisystem inflammatory syndrome following severe acute respiratory syndrome coronavirus 2 infection.
What is known and what is new?
• The mechanism of Multisystemic Inflammatory Syndrome in children (MIS-C) is not well understood up to now and the most that is known is from cases in adults, which has allowed us to propose that there is an effect on the endothelium associated with infection by viruses such as SARS-CoV-2.
• Children can develop a multisystem inflammatory syndrome and complications which requires early recognition and diagnosis to initiate timely treatment. We exposed additional information concerning the diagnosis and treatment of this important event
What is the implication, and what should change now?
• Neurological involvement must be taken into account in the evaluation of a patient with probable Multisystemic Inflammatory Syndrome in children (MIS-C), which requires early recognition and diagnosis to initiate timely treatment and obtain better results in children.
Introduction
Coronavirus disease 2019 (COVID-19), declared a pandemic by the World Health Organization (WHO) in March 2020, is caused by a virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). As of June 26, 2022, more than 543.5 million cases and more than 6.3 million deaths have been reported worldwide (2). Currently, although different vaccines are already available and even fourth doses have been administered, SARS-CoV-2 mutations may increase infectivity and therefore spread, diminish the protective effect of antibodies present after infection, vaccination, or antibody treatment, and may also increase the risk of infection (3), situations that are associated with the presence of fourth or fifth waves of contagion.
Since the beginning of the pandemic, it became evident that children infected with SARS-CoV-2 remain mostly asymptomatic or mildly symptomatic. In general, children with COVID-19 have a lower risk of hospitalization and life-threatening complications (4). However, some cases of severe disease or a post-infectious Multisystemic Inflammatory Syndrome in children (MIS-C) have been described (5,6). On the other hand, children with this complication may present with respiratory, gastrointestinal, lymphatic, hepatic, cardiac, or neurological symptoms or signs, although the latter are infrequent in children and were related to headache, syncope, convulsions, and seizures (7).
We present the case of an otherwise healthy 5-year-old boy who developed a multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection that progressed to ischemic cerebrovascular disease (8-13). Because acute ischemic stroke is uncommon in pediatric patients, there can be a delay in diagnosis leading to high morbidity and mortality. So, it is critical that clinicians are aware of the risk of stroke in MIS-C. We present this case in accordance with the CARE reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-22-43/rc).
Case presentation
A 5-year-old boy was admitted on January 21st, 2022 to the pediatric emergency department of a hospital in northern Peru with a productive cough and fever of 39 to 40 ℃ for the last 7 days. On the day of admission, bilateral eyelid edema, diffuse erythematous spots on different parts of the body, cracked lips, and joint pain was added, and he was taken to the hospital. The mother reported that a month ago she had mild respiratory symptoms (cough, rhinorrhea, febrile fever) that coincided with some cases of COVID-19 at school, and in those same days she had received the first dose of the Pfizer vaccine. The mother denies any history of head trauma in the child. On admission, tests were performed to rule out dengue (non-reactive) and a serological test for COVID-19, which was reactive for immunoglobulin G (IgG); other laboratory tests were performed: C-reactive protein (CRP) of 9.19 mg/dL, leukocyte count of 15,000/mm3 with deviation to the left, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (41.6 and 54.4 U/L respectively), and D-dimer of 6.37 µg/mL (Table 1).
Table 1
| Day/laboratory test | Admission | Day 3 | Day 9 | Reference values |
|---|---|---|---|---|
| Dengue rapid test | Non-reactive | – | – | – |
| COVID-19 antibodies | Reactive IgG; non-reactive IgM | – | – | – |
| PCR test for COVID-19 | 9.19 mg/dL | 23.13 mg/dL | 0.3 mg/dl | <2 mg/dL |
| Hemoglobin | 10.3 gr/dL | 9.3 gr/dL | 11.4 gr/dL | >11.5 gr/dL |
| Hematocrit | 30.8% | 28.2% | 34.3% | 35–42% |
| VCM | 76.7 fL | 77.4 fL | 79.4 fL | 75–87 fL |
| MCH | 25.7 pg | 25.5 pg | 26.5 pg | 25–33 pg |
| Leukocytes | 15,740/mm3 | 25,000/mm3 | 15,970/mm3 | 5,500–15,500/mm3 |
| Neutrophils | 29.59/mm3 | 21.04/mm3 | 9.74/mm3 | 1.5–8.5/mm3 |
| Lymphocytes | 0.66/mm3 | 2.51/mm3 | 5.43/mm3 | 2–8/mm3 |
| Platelets | 194,000/mm3 | 278,000/mm3 | 607,000/mm3 | 150,000–400,000/mm3 |
| PDW | 9.7 fL | 8.3 fL | 7.8 fL | 17.6 fL |
| PCT | 0.221% | 0.224% | 0.471% | <0.5% |
| AST | 41.6 UI/L | – | – | 10–50 UI/L |
| ALT | 54.4 UI/L | – | – | 5–45 UI/L |
| Dimero D | 6.37 µg/mL | – | – | <0.5 µg/mL |
| Fibrinogen | – | 507 mg/dL | – | 1.62–4.01 mg/dL |
| Thromboplastin time | 65 seconds | 46.70 seconds | 27 seconds | 33.6–43.8 seconds |
| Prothrombin time | 11.8 seconds | 10.80 seconds | 9.50 seconds | 12.1–14.5 seconds |
| Glucose | 82.4 mg/dL | 113 mg/dL | – | 60–100 mg/dL |
| Urea | – | 14 mg/dL | – | 6–20 mg/dL |
| Complete urine test | Scanty epithelial cells and mild proteinuria | Scarce epithelial cells and mucoid filament in regular quantity | – | – |
COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; VCM, medium corpuscular volume; MCH, medium corpuscular hemoglobin; PDW, platelet distribution width; PCT, procalcitonin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; IgG, immunoglobulin G; IgM, immunogobulin M.
Physical examination revealed an irritable child with a heart rate of 162 beats per minute, respiratory rate of 50 breaths per minute, and oxygen saturation of 93% on room air. In addition, he had dry oral mucous membranes, raspberry tongue, conjunctival injection, generalized exanthema with a polymorphous pattern, cervical and axillary lymphadenopathy; and chest auscultation showed rhonchi in both lung fields. The rest of the examination was unaltered.
Other laboratory tests were performed and yielded hemoglobin 10.3 g/dL, glucose 150 mg/dL, leukocyte count 31,000/mm3 with left shift, and CRP 10.65 mg/dL (Table 1). Cholesterol, triglycerides, total protein, albumin, bilirubin, and liver enzymes, creatinine, urea, glucose and electrolytes, urine examination, electrocardiogram, abdominal ultrasound, blood gas analysis and chest X-ray were reported within normal. No more respiratory tests were considered to perform. Echocardiography reported mild left atrial dilatation and laminar pericardial effusion without hemodynamic compromise. From the beginning, a diagnosis of MIS-C was proposed, and treatment with human immunoglobulin, methylprednisolone, ceftriaxone 1 g IV e//24 hours, and oxygen by binasal cannula was initiated (Figure 1).
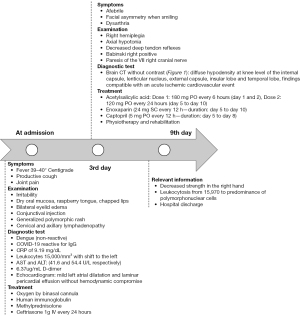
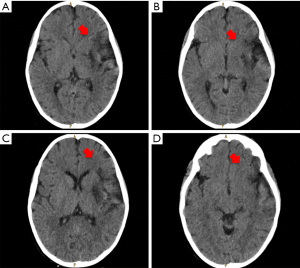
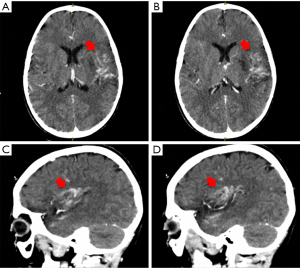
On the third day of hospitalization, he no longer presented fever, however, the mother reported that the child presented facial asymmetry when smiling. On examination it was found: right hemiplegia, axial hypotonia, diminished osteotendinous reflexes, positive right Babinski, dysarthria, and paresis of the right VII cranial nerve. A brain CT scan without contrast was requested (Figure 2) and diffuse hypodensity was found at the level of the knee of the internal capsule, lenticular nucleus, external capsule, insula lobe, and temporal lobe, in addition, asymmetry of lateral ventricles, the smaller size of the left ventricle and effacement of the left Sylvian fissure, findings compatible with acute ischemic cardiovascular event. A control CT scan with contrast three days later showed the same alterations (Figure 3), the AngioTEM performed later did not provide further information.
Based on the diagnosis of acute ischemic stroke, treatment was started with acetylsalicylic acid: Dose 1: 180 mg PO every 6 hours (day 1 and 2), Dose 2: 120 mg PO every 24 hours (day 5 to day 10), enoxaparin [24 mg SC every 12 h—duration: day 5 to day 10], captopril (5 mg PO every 12 h—duration: day 5 to day 8), physiotherapy and rehabilitation. In the following days, the evolution is good. He was discharged with remarkable clinical improvement; in his outpatient controls, only a slight decrease of strength in the right hand was evidenced.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The COVID-19 pandemic has affected millions of people worldwide. In most of the cases, children have milder clinical symptoms and better prognosis than the adult population (14). However, since April 2020, reports from the United Kingdom reported a clinical presentation in children similar to Kawasaki disease, but not necessarily meeting all established criteria for Kawasaki diagnosis, determining it as Kawasaki-Like or Kawasaki incomplete (15). Severe COVID-19 generally occurs in the initial and acute phase of infection with the SARS-CoV-2 virus. Severe COVID-19 is contagious. MIS-C commonly occurs three to six weeks after a mild or asymptomatic case of COVID-19 or an exposure to someone who has had COVID-19. MIS-C is not contagious. Kawasaki disease is an illness that has no known cause. It is suspected that exposure to a virus (not necessarily SARS-CoV-2) may trigger the condition, but this has not been confirmed. Kawasaki disease is not contagious (16,17). The relationship among Kawasaki and coronavirus is not yet specific, however, it has been associated with an altered immune response against the virus in a picture mediated by an excessive cytokine storm after acute infection; this condition has been called MIS-C, pediatric hyperinflammatory syndrome or pediatric multisystem inflammatory syndrome associated with SARS-CoV-2 (PIMS-CoV-2) (10). Most children with this condition have a positive serology for SARS-CoV-2 predominantly IgG, with a negative PCR test (as in the case of our patient), strengthening the theory of an irregular immune process triggered after an acute infection (18). However, there are reports of children with positive serology and PCR test, or with both tests negative (18-20). To date, the time to onset of MIS-C ranges from one to several weeks after acute infection (21).
The operational definition of this disease is characterized by presenting with fever >38 degrees for at least 3 days, with at least 2 of the following criteria: conjunctival rash, mucosal inflammation, myocardial dysfunction and/or hypotension, coagulopathies (prolonged prothrombin, thromboplastin activated and elevated D-dimer), gastrointestinal disturbances; in addition, there must be elevated inflammatory markers, predominantly C-reactive protein, evidence of SARS-CoV-2 infection (either with a rapid test, predominantly IgG, molecular test or epidemiological contact) and absence of another cause that justifies the inflammatory process (22). In this case report, the patient met all the criteria mentioned above (except hypotension and gastrointestinal problems), in addition, the association with coronavirus infection is reaffirmed by the history of epidemiological contact, respiratory symptoms described by the mother, and serology with a predominance of IgG, as well as the age of presentation in children older than 5 years compared to the classic that prevails in children under 5 years, in addition, there is greater cardiac involvement and especially high elevation of acute phase reactants (23).
Then, as mentioned above, during the pandemic period, MIS-C was reported, with characteristics similar to Kawasaki disease. However, the mechanism of MIS-C in the context of COVID-19 is not well understood. It has been suggested that is the result from an abnormal I macrophage activation syndrome and cytokine release syndrome. Among the clinical features of the case reports and reviews, there was fever, rash, dilation of conjunctival blood vessels, cervical lymphadenopathy, redness of the oropharynx, and neurological symptoms such as headache and altered mental status (24,25). It was reported that as much as there were similarities, differences could also be found, such as a higher incidence of gastrointestinal symptoms, myocarditis, shock, and coagulopathy (24).
The complications reported in MIS-C are classified according to the organ affected, being found at the cardiovascular level; myocarditis, pericarditis, shock, coronary artery anomalies, among others; at the respiratory level; pulmonary nodules, pleural effusion, empyema, etc.; and at the nervous system level, behavioral changes and irritability (13–35%), aseptic meningitis, peripheral facial nerve palsy and cerebrovascular accidents (3%) have been reported (25,26), the latter being the affectation presented by our patient.
The pathophysiology of acute ischemic stroke in MIS-C has not yet been fully elucidated, however, what is known in adults has allowed us to propose that there is an effect on the endothelium associated with SARS-CoV-2 infection in different organs, mainly in the lung, but also in the kidney, heart, intestine and also in the cerebral vessels (27). Possible mechanisms that can trigger ischemic cerebrovascular event (CVE) could be post-infectious immune processes, direct infection of the central nervous system (CNS) by the virus, and virus-induced hyperinflammatory and hypercoagulable states. Hemorrhagic and ischemic strokes, encephalitis, meningitis, acute disseminated encephalopathy, endothelitis, and venous sinus thrombosis are some cases of COVID-19 CNS disease (28). Although an immune-mediated inflammatory response stimulated by macrophages, neutrophils, and monocytes, followed by antibody production by plasma and B cells, is generated in children, especially in those with MIS-C late after the initial infection, this appears to be more likely pathogenesis than the direct viral invasion of tissues (8). Patients benefit from intravenous immunoglobulin, methylprednisolone, and enoxaparin, although some patients require thrombolysis and even surgery. The literature reports a few cases of patients with MIS-C and debuting with acute ischemic CVE, some of them achieving full recovery, others with sequelae, and others who died (Table 2) (8-13).
Table 2
| Author, year | Country | Type of study | Sample | Diagnostic method | Type of stroke | Type of treatment | Treatment results |
|---|---|---|---|---|---|---|---|
| Chang et al., 2022 (8) | United States | Case series | 2 | Brain CT Agio CT of the head and neck | Ischemic stroke | IVIG 2 g/kg and IV methylprednisolone 2 mg/kg/day ×5 days, followed by a gradual reduction of oral prednisone for 3 days and therapeutic enoxaparin 1 mg/kg twice daily | Patient 1: subtle right hemiparesis that resolved on day 30. Patient 2: complication: DVT in the right lower extremity associated with a catheter. Mild expressive aphasia and mild right hemiparesis |
| Shala et al., 2021 (9) | Kosovo | Case Report | 1 | MRI and MRA of the brain | Ischemic stroke (terminal branches of the left middle cerebral artery) | IVIG (2 g/kg) along with low dose aspirin therapy and methylprednisolone 40 mg twice daily | The complete resolution, except for discrete right-sided hemiparesis (4/5 muscle strength) |
| Coronado Munoz et al., 2022 (10) | Peru | Cases and controls | 47 | Brain CT | 5 hemorrhagic strokes and 1 ischemic stroke | Intravenous immunoglobulins. Low-dose methylprednisolone (does not refer to exact dose) | 4 of 6 patients died. One patient with hemorrhagic stroke and one patient with ischemic stroke survived, but had significant sequelae |
| Salik et al., 2021 (11) | United States | Case Report | 1 | Brain CT; magnetic resonance imaging | Right basal ganglia ischemic stroke with hemorrhagic transformation | IVIG 2 g/kg, methylprednisolone 2 mg/kg/day, and anakinra for suspected COVID-19 cytokine storm | Mental status gradually improved throughout the hospital stay, although the patient had residual left hemiparesis |
| Scala et al., 2022 (12) | Italy | Case Report | 1 | CT and MRI of the skull | Ischemic stroke: ischemia of the right middle cerebral artery | Heparin and acetylsalicylic acid bridging systemic thrombolysis followed by endovascular thrombectomy. Emergency DHC | Neurological examination at discharge was Glasgow 14 with persistent severe left-sided hemiparesis |
| Tiwari et al., 2021 (13) | India | Case Report | 1 | Non-contrast computed tomography and cerebral CT angiography | Ischemic stroke | IV immunoglobulin (2 g/kg for 2 days), methylprednisolone (30 mg/kg IV for 5 days), dexamethasone (0.15 mg/kg per day for 2 weeks), remdesivir (5.0 mg/kg by IV infusion over 1 hour, followed by 2.5 mg/kg per day by IV infusion for 5 days), and low molecular weight heparin (1 mg/kg SC twice daily for 2 weeks followed by once daily for 1 week) | His clinical status improved progressively, and mechanical ventilation was withdrawn on day 12. His serum CRP, ferritin, and lactate dehydrogenase values returned to normal |
CT, computerized tomography; MRI, magnetic resonance imaging; MRA, magnetic resonance angiography; IVIG, intravenous immunoglobulin; IV, intravenous; COVID-19, coronavirus disease 2019; DHC, decompressive right hemicraniectomy; SC, subcutaneous; DVT, deep venous thrombosis; CRP, C-reactive protein.
In the present investigation, a rare and unusual presentation syndrome is reported. Therefore, through the present review, we exposed additional information concerning the diagnosis and treatment of this important event. However, its usefulness for medical decision-making is still limited, so we hope it will be a valuable resource of new information that can encourage and serve in the future to carry out research studies with a higher level of evidence.
Conclusions
The pandemic of COVID-19 continues in all countries, with fourth, fifth, and sixth waves; and with the appearance of new variants. Infections in children are rare, however, they can occur and very few of them develop a complication called MIS-C. In this scenario, although very rare, few cases of ischemic CVE have been reported, such as the one we present. In this sense, this neurological involvement should be taken into consideration, which requires early recognition and diagnosis to initiate timely treatment and obtain better results in children.
Acknowledgments
We are grateful to all of those with whom we have had the pleasure to work during this and other related projects.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-22-43/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-22-43/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Forchette L, Sebastian W, Liu T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr Med Sci 2021;41:1037-51. [Crossref] [PubMed]
- COVID-19 Map - Johns Hopkins Coronavirus Resource Center n.d. Available online: https://coronavirus.jhu.edu/map.html (accessed August 19, 2022).
- Hemmer CJ, Löbermann M, Reisinger EC. COVID-19: epidemiology and mutations: An update. Radiologe 2021;61:880-7. [Crossref] [PubMed]
- Nikolopoulou GB, Maltezou HC. COVID-19 in Children: Where do we Stand? Arch Med Res 2022;53:1-8. [Crossref] [PubMed]
- Ashraf S, Abbasi FS, Atiq M. Kawasaki Shock Syndrome and Covid-19. J Coll Physicians Surg Pak 2021;31:135-7. [Crossref] [PubMed]
- Giacalone M, Scheier E, Shavit I. Multisystem inflammatory syndrome in children (MIS-C): a mini-review. Int J Emerg Med 2021;14:50. [Crossref] [PubMed]
- Kornitzer J, Johnson J, Yang M, et al. A Systematic Review of Characteristics Associated with COVID-19 in Children with Typical Presentation and with Multisystem Inflammatory Syndrome. Int J Environ Res Public Health 2021;18:8269. [Crossref] [PubMed]
- Chang J, Bulwa Z, Breit H, et al. Acute Large Vessel Ischemic Stroke in Patients With COVID-19-Related Multisystem Inflammatory Syndrome. Pediatr Neurol 2022;126:104-7. [Crossref] [PubMed]
- Shala N, Jashari F, Boshnjaku D, et al. A 14-Year-Old Male Patient with Kawasaki Disease Presented with Stroke after COVID-19. Case Rep Infect Dis 2021;2021:5576440. [Crossref] [PubMed]
- Coronado Munoz A, Tasayco J, Morales W, et al. High incidence of stroke and mortality in pediatric critical care patients with COVID-19 in Peru. Pediatr Res 2022;91:1730-4. [Crossref] [PubMed]
- Salik I, Jacoby M. Carotid Artery Dissection and Hemorrhagic Stroke in the Setting of Multisystem Inflammatory Syndrome in Children. Cureus 2021;13:e13640. [Crossref] [PubMed]
- Scala MR, Spennato P, Cicala D, et al. Malignant cerebral infarction associated with COVID-19 in a child. Childs Nerv Syst 2022;38:441-5. [Crossref] [PubMed]
- Tiwari L, Shekhar S, Bansal A, et al. COVID-19 associated arterial ischaemic stroke and multisystem inflammatory syndrome in children: a case report. Lancet Child Adolesc Health 2021;5:88-90. [Crossref] [PubMed]
- Sood M, Sharma S, Sood I, et al. Emerging Evidence on Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 Infection: a Systematic Review with Meta-analysis. SN Compr Clin Med 2021;3:38-47. [Crossref] [PubMed]
- Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395:1607-8. [Crossref] [PubMed]
- Webinar May 19, 2020 - Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) n.d. Available online: https://emergency.cdc.gov/coca/calls/2020/callinfo_051920.asp (accessed August 19, 2022).
- Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 3. Arthritis Rheumatol 2022;74:e1-e20. [Crossref] [PubMed]
- Giraldo-Alzate C, Tamayo-Múnera C, López-Barón E, et al. Síndrome inflamatorio multisistémico en niños asociado a COVID-19. Revisión narrativa de la literatura a propósito de un caso. Acta Colombiana de Cuidado Intensivo 2022;22:137-48.
- Coll-Vela LE, Zamudio-Aquise MK, Nuñez-Paucar H, et al. COVID-19-associated multisystem inflammatory syndrome in children: case series at a pediatric hospital in Peru. Rev Peru Med Exp Salud Publica 2020;37:559-65. [Crossref] [PubMed]
- Belhadjer Z, Méot M, Bajolle F, et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation 2020;142:429-36. [Crossref] [PubMed]
- Aldawas A, Ishfaq M. COVID-19: Multisystem Inflammatory Syndrome in Children (MIS-C). Cureus 2022;14:e21064. [Crossref] [PubMed]
- Hennon TR, Yu KOA, Penque MD, et al. COVID-19 associated Multisystem Inflammatory Syndrome in Children (MIS-C) guidelines; revisiting the Western New York approach as the pandemic evolves. Prog Pediatr Cardiol 2021;62:101407. [Crossref] [PubMed]
- Zhang QY, Xu BW, Du JB. Similarities and differences between multiple inflammatory syndrome in children associated with COVID-19 and Kawasaki disease: clinical presentations, diagnosis, and treatment. World J Pediatr 2021;17:335-40. [Crossref] [PubMed]
- Sharma C, Ganigara M, Galeotti C, et al. Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison. Nat Rev Rheumatol 2021;17:731-48. [Crossref] [PubMed]
- Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol 2020;20:453-4. [Crossref] [PubMed]
- Rife E, Gedalia A. Kawasaki Disease: an Update. Curr Rheumatol Rep 2020;22:75. [Crossref] [PubMed]
- Uginet M, Breville G, Hofmeister J, et al. Cerebrovascular Complications and Vessel Wall Imaging in COVID-19 Encephalopathy-A Pilot Study. Clin Neuroradiol 2022;32:287-93. [Crossref] [PubMed]
- Jha NK, Ojha S, Jha SK, et al. Evidence of Coronavirus (CoV) Pathogenesis and Emerging Pathogen SARS-CoV-2 in the Nervous System: A Review on Neurological Impairments and Manifestations. J Mol Neurosci 2021;71:2192-209. [Crossref] [PubMed]
Cite this article as: Caballero-Alvarado J, Rodríguez Millones K, Ruiz Gonzales M, Rojas Alvarado AB, Zavaleta Corvera C, Barboza JJ. Ischemic stroke associated with Multisystemic Inflammatory Syndrome in children after SARS-CoV-2 infection: a case report. Pediatr Med 2023;6:40.

