
Oxygenation in the newborn: a narrative review in Chinese perspective※
Introduction
Oxygen therapy is a common treatment to correct hypoxemia caused by various reasons. The goal of oxygen therapy is to improve alveolar oxygen exchange and increase arterial oxygen partial pressure, thereby preventing hypoxic injury to tissues and organs. The understanding of the effects of oxygenation on the newborn infants has been evolving over time. The detrimental effects of hypoxemia on neonatal mortality and tissue/organ damage resulting in organ dysfunction, especially poor neurodevelopmental outcomes have been well described. In recent years, more attention has been paid to the adverse effects of hyperoxia on developing neonates, particularly preterm infants. With the discovery of oxygen toxicity and related long-term health consequences, e.g., lung injury and retinopathy of prematurity (ROP), oxygen is now viewed as a “drug” and its use in the neonates has changed from liberal use in the 1960s to more restrictive use in the recent years.
The level of neonatal care, especially neonatal intensive care in China has been developing very rapidly over the past two decades. As a result, there has been a rapid and sustained decline in neonatal mortality rate over this period of time, from 22.10 per 1,000 live births in 1999, to 9.23 in 2009, and then to 3.46 in 2020 (1). Survival of very preterm infants has also increased. The Chinese Neonatal Network (CHNN) reported a survival rate of 95.4% of the infants born at <32 weeks’ gestation who received a full course of postnatal care in the network neonatal intensive care units (NICU) in 2019. Among these infants, 57.2% survived without major morbidity (2). Oxygen therapy and management of neonatal oxygenation have also been evolving in China during this time. The objectives of this review are (I) reviewing the evolution and, (II) discussing the current practices of neonatal oxygenation management in China. We present this article in accordance with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-22-52/rc).
Methods
This review summarized data from published articles over the past 30 years. Common databases, including PubMed, MEDLINE, Embase, Chinese databases (medlive.cn, cnki.net, and medbooks.com.cn) and other relevant sources including Google and Baidu search engines, and World Health Organization websites were used to search relevant articles from 1992 to 2022. Keywords used for the search are listed in Table 1, and an example of search strategy of one database is presented in Table S1.
Table 1
| Items | Specification |
|---|---|
| Date of search | August 10, 2021, and September 20, 2022 |
| Databases and other sources searched | • PubMed, MEDLINE, Embase, Chinese databases (medlive.cn, cnki.net, and medbooks.com.cn) • Other relevant sources including Google and Baidu search engines • World Health Organization websites |
| Search terms used | Newborn, neonate, premature infants, preterm, birth asphyxia, perinatal asphyxia, oxygenation, hypoxia, hyperoxia, oxygen saturation, pulse oximetry, near infrared spectroscopy, transcutaneous PO2 monitoring |
| Timeframe | 1992–2022 |
| Inclusion and exclusion criteria | • Inclusion: any clinical research and review manuscripts in English and Chinese • Exclusion: animal studies or human subjects over 1 year of age |
| Selection process | • Y.S. and X.R. conducted initial independent search and reached agreement of included manuscripts • H.Z. made final decision of included studies |
Discussion
Oxygenation management in the delivery room
In response to the United Nation Millennium Developmental Goal of reducing child mortality, the Chinese Ministry of Health organized the Neonatal Resuscitation Program Task Force in 2003 (3). With the help of Chinese professional organizations (the Chinese Society of Perinatal Medicine, the Chinese Pediatric Society, and the Chinese nursing Association) and American Academy of Pediatrics, using the US Neonatal Resuscitation Program (NRP) textbook and training methods as the blueprint, nationwide NRP training started in 2004. Within the 10-year period of 2005–2015, NRP spread around the country resulting in decreased rate of birth asphyxia from 3.08% in 2003 to 2.76% in 2006, 2.33% in 2010 and then to 1.79% in 2014. Consequently, there was a progressive decline in the mortality from birth asphyxia 3.08 per 10,000 live births in 2003 to 1.64 per 10,000 live births in 2014 (4-7).
In addition to improved resuscitation skills of the perinatal care team, standardization of neonatal resuscitation also resulted in rapid improvement in basic resuscitation equipment, including bag and mask resuscitators, tracheal intubation equipment and radiant warmers. Over this period, the availability of equipment for oxygen therapy and monitoring also progressed but at a slower pace. The Chinese neonatal resuscitation guidelines recommend monitoring preductal pulse oxygen saturation in patients with cyanosis or supported with positive pressure ventilation. A survey of 347 delivery hospitals in China found that by November 2015, over 90% of the hospital delivery rooms were equipped with basic neonatal resuscitation equipment. However, the availability of pulse oximeters (87.6%) and oxygen blenders (31.8%) varied greatly, with much less availability in lower-level hospitals. In the 163 county level hospitals, 73.1% hospitals were equipped with pulse oximeters, however only 2.5% of these hospitals had oxygen blenders in the delivery room (7). In an international survey of delivery room oxygen management for moderate-to-late preterm infants conducted between October 2020 and March 2021, 81.5% of the 148 neonatologists in China responded to the survey reported that pulse oximeter is available in both delivery room and operating room. This percentage is much lower (41.8%) for oxygen blenders. 11% and 35.6% of neonatologists reported that neither pulse oximeter nor oxygen blender was available in their hospital delivery room or operating room (8).
The current Chinese NRP textbook and most recent 2021 revised recommendation (9,10) both listed pulse oximeters and oxygen blenders as necessary neonatal resuscitation equipment. Oxygen delivering equipment (mask, tubing, flow inflation bags or T-piece resuscitators) should be set up prior to delivery of high risk infants, with oxygen flow set at 5–10 L/min through an oxygen blender and oxygen concentration at 21% for a term delivery or late preterm newborns [≥35 weeks gestational age (GA)], and 21–30% for preterm deliveries. Oxygen concentration should be titrated during resuscitation to achieve the target oxygen saturation value based on pulse oximeter monitoring. However, 100% oxygen should be avoided in term and late preterm newborns ≥35 weeks GA since it is associated with excess mortality (11). These recommendations are in congruent with the most recent American NRP recommendations (12,13).
In the resource limited settings where oxygen blender is not available, the 2021 Chinese NRP guideline recommended using self-inflation bags to achieve 4 different oxygen concentration: 21% when the bag is not connected to an oxygen source, 40% when connected to an oxygen source without oxygen reservoir, 90% when connected to an oxygen source with an tube-shaped oxygen reservoir, and 100% with a bag-shaped reservoir (10).
The ideal oxygen saturation (SpO2) after birth, i.e., not too high causing oxygen toxicity and not too low resulting in hypoxic injury, has not been established and is an ongoing topic of controversy. The current target pre-ductal SpO2 in the first 10 minutes after birth recommended by the US NRP guidelines were selected based on a consensus of acceptable values derived from data obtained from healthy, term babies breathing room air (14). Two studies reported data from Chinese newborns, one reported median level and percentile range within the first 10 minutes of life at sea level (15), and the other reported data from higher altitude in the first 30 minutes of life (16). As shown in Table 2, although the SpO2 level is slightly lower at altitude ≥3,000 meters in the first 3 minutes of time, these data suggest that it is reasonable to adopt the target SpO2 range recommended by AAP in the Chinese population.
Table 2
| Time | NRP target range % | Sea-level study %, median (P10–P95) |
High-altitude study %, mean ± SD | ||||
|---|---|---|---|---|---|---|---|
| Vaginal delivery | C-section | 2,000–3,000 m | 3,000–4,000 m | >4,000 m | |||
| 1 min | 60–65 | 72 (62–85) | 70 (62–85) | 61±4 | 59±4 | 56±4 | |
| 2 min | 65–70 | 77 (65–90) | 73 (62–86) | 65±3 | 64±3 | 62±3 | |
| 3 min | 70–75 | 83 (70–95) | 75 (65–88) | 70±3 | 69±3 | 67±2 | |
| 4 min | 75–80 | 91 (78–98) | 78 (63–91) | 76±3 | 75±3 | 74±2 | |
| 5 min | 80–85 | 92 (80–98) | 80 (66–95) | 80±2 | 80±2 | 78±2 | |
| 10 min | 85–95 | 98 (92–98) | 96 (82–98) | 90±2 | 88±3 | 86±2 | |
NRP, Neonatal Resuscitation Program.
More controversies exist regarding what inspired oxygen concentration should be used for the initiation of resuscitation or the target SpO2 range of preterm infants. Several studies demonstrated similar or better clinical outcomes (i.e., achieving targeted saturations and heart rates in the first 10 minutes of life, similar mortality, improved neurodevelopmental outcomes, less biomarkers of oxidative stress, decreased incidence of BPD) in preterm infants resuscitated with 21–30% initial inspired fraction of oxygen (FiO2) as compared to higher FiO2 of 60–100% (17-20). However, some other studies reported potential harm of lower initial oxygen with increased risks of death of severe neurologic injury, especially in extremely preterm infants (21-23). In addition, the post hoc exploratory analyses of the randomized controlled Torpido trial, comparing clinical outcomes of preterm infants resuscitated with room air vs. 100% oxygen, found that infants who did not achieve SpO2>80% at 5 minutes after birth were more likely to die or have neurodevelopmental impairment (NDI) (OR 1.85, 95% CI: 1.07–3.2, P=0.03). Therefore, large and robust trials examining oxygen use for preterm infants are needed. Regardless of these controversies, limited availability of oxygen blenders and pulse oximeters makes titrating oxygen concentration not possible, and potentially put preterm infants at higher risk of exposure to hyperoxia or hypoxemia. More efforts need to be spent to better equip the Chinese hospitals with these essential oxygen delivery and monitoring devices, especially in the lower lever hospitals and in rural areas.
Postnatal oxygenation management in the NICUs
Oxygen therapy plays an important role in the care of critically ill neonates. The goal of oxygen therapy is to deliver sufficient oxygen to the tissues while minimizing oxygen toxicity and oxidative stress. However, the optimal oxygenation management in the NICUs, especially for preterm infants, is unknown and has been a hot topic of research in recent years.
Oxygenation management of critically ill infants
Oxygen therapy is often needed in infants with respiratory diseases, especially those with hypoxic respiratory failure. Proper use of positive pressure ventilation, including continuous positive airway pressure (CPAP), nasal intermittent positive pressure ventilation (NIPPV), or mechanical ventilation is critical in achieving good oxygenation in these infants. Proper use of CPAP or positive end expiratory pressure (PEEP) have a direct impact on oxygenation by achieving “open lung” ventilation, and therefore, decreasing ventilation-perfusion mismatch. The Subspecialty Group of Neonatology of the Society of Pediatrics Chinese Medical Association has recommended starting CPAP in patients on >30% FiO2 with PaO2 <50 mmHg or SpO2 <90%, and starting intubated mechanical ventilation in patient on 60–70% FiO2 with PaO2 <50–60 mmHg or SpO2 <85% (24).
With the advance in respiratory support technologies, the use of oxygen hood (oxyhood) has been decreasing in western countries. However, it is still being utilized in many NICUs in China. Disadvantages of oxyhood include it cannot provide any flow or positive pressure support, and accurate oxygen delivery is difficult. Song and He measured the effects of various flow rate on oxygen delivery and PCO2 in term infants. They found that the delivered oxygen concentration and patient PCO2 levels varied with varying flow and size of oxyhood (25). In recent years, humidified high flow nasal canular (HHFNC) has become increasingly popular in the Chinese NICUs for term infants with respiratory distress. In a study of term infants with respiratory failure due to pneumonia, Xu et al. compared HHFNC of 2–10 L/min with CPAP 5–7 cmH2O. Both groups started with FiO2 of 30–40% and titrated the support to maintain pH at 7.2–7.4, PCO2 at 40–55 mmHg, and PaO2 at 50–80 mmHg. Both groups showed improvement in respiratory distress, PCO2 and PaO2 24 hours after the initiation of treatment. However, the HHFNC group had faster improvement in clinical symptoms and shorter duration of hospital stay (26).
Data from adult and pediatric patients with acute respiratory distress syndrome (ARDS) suggested prone positioning may help optimizing ventilation/perfusion match and improve oxygenation. Unfortunately, data in the neonatal population is limited. In an interventional analytic study of 69 premature infants with RDS, Eghbalian reported that oxygen saturation was significantly higher in the prone compared with the supine position (27). In one study of 80 neonates with respiratory failure requiring Bi-level CPAP treatment from China, Tang et al. demonstrated improve heart rate and PaO2/FiO2 ratio 24 hours after prone positioning as compared to patients remained on supine position. However, the details of patient demographics and method of intervention was not provided in the article (28). More high-quality research is needed to further elucidate the effects of prone positioning on neonatal oxygenation.
Inhaled nitric oxide (iNO), in combination with good ventilatory support, is the main state treatment for neonatal hypoxic respiratory failure in the western countries. However, iNO is still not widely available in China and other vasodilators (such as sildenafil and bosentan) are frequently used instead (29,30). When medical managements have failed, extracorporeal membrane oxygenation (ECMO) is the last resort but effective treatment for neonatal hypoxic respiratory failure. Although still in the developing phase, neonatal ECMO has been developing fast over the last 10 years in China (31). However, there are still many challenges that hinders the development of neonatal ECMO in China, including issues with equipment, system management, team training, and multi-disciplinary support (32).
Oxygenation management of preterm infants
The advance in neonatal intensive care has resulted in the increased survival of extremely preterm infants born at less than 28 weeks’ gestation. These infants at extreme prematurity are at increased risk of death and major morbidities such as ROP, brain injury causing long-term neurodevelopmental impairments (NDI), bronchopulmonary dysplasia (BPD), and necrotizing enterocolitis (NEC). Previous studies have demonstrated that unrestricted, unmonitored oxygen therapy has potential harms without clear benefits (33). However, the optimal level of oxygenation to maximize the survival of these extremely preterm infants without incurring significant morbidity is unknown and has been the topic of investigation for decades. To answer this question, the NeOProM (Neonatal Oxygenation Prospective Meta-analysis) Collaboration was formed in 2003 and subsequently carried out a planned meta-analysis of data from 5 large international multi-center prospective clinical trials. These trials enrolled infants born before 28 week’s gestation and compared clinical outcomes of targeting two different SpO2 ranges: lower (85–89%) vs. higher (91–95%). Although there was no difference in the primary outcome of death or major disability at 18–24 months corrected age, the lower SpO2 target range was associated with a higher risk of death and NEC, but a lower risk of ROP requiring treatment (34).
Based on the results of these well-designed trials, and pending additional data from the NeOProM, in 2016, AAP concluded that a targeted oxygen saturation range of 90% to 95% may be safer than 85% to 89% at least for some infant. In addition, an upper alarm limit of approximately 95% while the infant remains on supplemental oxygen, and a lower alarm limit extend somewhat below the lower target chosen by the institution based on practical and clinical considerations were suggested (35). In the same year, the updated European Consensus Guidelines on the Management of Respiratory Distress Syndrome recommended that the saturation target should be between 90% and 94% in preterm babies receiving oxygen, with an alarm limit of 89% to 95% (36).
Neonatologists in China have been following the development of international guidelines in oxygenation management for preterm infants very closely. Over the years, several clinical guidelines or consensus statements have been released from different Chinese professional associations regarding to the care for preterm infants. A guideline for clinical application of NIPPV in preterm infants recommends that FiO2 should be titrated to maintain the target oxygen saturation at 90–94% (37), while another guideline for neonatal mechanical ventilation points out that the target should be 90–95% (24). Both guidelines are in consistent with the AAP and European guidelines. However, neither suggested the alarm limits.
ROP is a serious complication of prematurity. Well described risk factors of ROP include low GA and birth weight, and oxygen exposure (38-40). The reported incidence of ROP in China varies based on different screening criteria used. A multi-center survey study, which enrolled 14,015 infants born at less than 34 weeks’ gestation in 25 NICUs in China between 2015 and 2018 reported an incidence of ROP of 22.5% in preterm infants born at <32 weeks’ GA, 16.4% in those born at <34 weeks’ GA and 18.0% in those born at <2,000 g. However, the incidence of ROP was significantly higher in infants born at <28 weeks’ GA (56.5%) and <1,000 g (54.2%) (41). Another study from north China reported an incidence of 17.9% in infants born at <35 weeks GA or <2,300 g (42). The ROP screening guideline by the Chinese Ophthalmological Society calls for routine screening for ROP in infants born at <32 weeks GA or with birth weight <2,000 g, whereas the guideline by the Chinese Medical Doctor Association recommends screening all infants born at ≤34 weeks GA or with BW <2,000 g (43). Both guidelines suggest extending the screening to older and larger preterm infants who were critically ill or received oxygen therapy. In some areas in China (e.g., Guangdong province), ROP screening has been extended to include all preterm infants born at <37 weeks GA or weighted less than 2,500 g due to the concerns of missing timely diagnosis and treatment of ROP in the older and larger preterm infants. In an outpatient study, Mao et al. reported 4.26% of preterm infants born at 2,000–2,500 g had ROP. Of these 2 patients required treatment (44). However, these extended screening criteria bring significant workload to the pediatric ophthalmologists in China. In a retrospective cohort study of 5,606 infants from 4 tertiary NICUs in Shanghai, Yang et al. demonstrated that 1,619 less infants would need to be screened if the screening criteria change from GA <32 weeks or BW <2,000 g to GA <32 weeks or BW <1,600 g. However, a screening criterion of GA <31 weeks or BW <1,500 g, which is similar to the US screening criteria of GA <30 weeks or BW <1,500 g, only had a sensitivity of 85.1% (45). Although the incidence is low, data from China does bring up the concerns for ROP in the relatively older and larger preterm infants. Similar concerns have been raised by investigators in Turkey. In a retrospective observational study of 543 infants born at 32–35 weeks GA, Tiryaki Demir et al. reported an overall incidence of ROP of 13.1%, and 2.6% required treatment due to severe ROP (46). However, the etiology of ROP in these infants is not very clear and could be related to improper respiratory management (e.g., unblended oxygen use) causing hyperoxia or swings in oxygenation in the early postnatal life.
The association between oxygen therapy and the development of ROP has been well documented (40). In addition, recent prospective observational or clinical trial studies in the US extremely premature infants have demonstrated increased risks of severe ROP in infants with more fluctuation of oxygenation in the first 4 weeks of life, or with higher percentage of time at 91–96% SpO2 in postnatal week 1–5 for infants who spent at least 2 weeks on oxygen and at 97–100% SpO2 in postnatal week 6–9 for infants who spent at least 3 weeks on oxygen (47,48). Given this association, the initial guideline of oxygen therapy in preterm infants for the prevention and treatment of ROP in China was published in 2004 and subsequently revised in 2016. This revised guideline emphasized that FiO2, PaO2 and SpO2 should be closely monitored during oxygen treatment to prevent ROP. If monitoring could not be performed due to a lack of equipment, patient should be transferred to hospitals that are adequately equipped. The guideline suggested that oxygen therapy should be initiated in preterm infants with respiratory distress, PaO2 <50 mmHg or SpO2 <85% on room air. Ideal targets of oxygen therapy are to maintain PaO2 between 50 and 80 mmHg or SpO2 between 88–93%, and avoid SpO2 >95% (43). Unfortunately, these ideal targets are difficult to achieve in many NICUs in China for several reasons: (I) oxygen blenders may not be available (especially in lower level NICUs) or may not be in sufficient quantity even in large tertiary NICUs. As a result, unblended oxygen given as free-flowing oxygen in the incubators, via nasal cannula or through oxyhood are still common practices in the Chinese NICUs. (II) Difficulties with the monitoring of PaO2 and SpO2 in extremely preterm infants. (III) Timely titration of oxygen based on SpO2 is hard to implement due to the low nurse-patient ration and high workload. (IV) Policies for maintaining target oxygen saturation and alarm settings for very preterm infants are not established in many NICUs. Therefore, despite the Chinese recommendations of maintaining SpO2 between 90–95% in the very preterm infants receiving oxygen therapy, the actual time they spend at target SpO2 range is unknown in most of Chinese NICUs. During baseline data acquisition for a quality improvement project in our center, we found that in preterm infants born at ≤32 weeks’ GA, the average time spent in the target 90–94% range was <26% and over 60% of time were spent with SpO2 ≥95%.
Although difficult, efforts have been made to achieve the target oxygenation as recommended by the Chinese guidelines. In a case control study, Guo et al. found that the duration of oxygen therapy is an independent risk factor for severe ROP and frequent use of mechanical ventilation increased the incidence of severe ROP (49). In a retrospective case series study of 166 extremely low birth weight (ELBW) infants, oxygen therapy was strictly controlled to limit oxygen exposure and to maintain PaO2 at 50–80 mmHg or SpO2 at 90–95%. Although the authors reported similar incidence of ROP (56.6%) to the incidence of ROP in ELBW infants (53–66%) reported by other studies in China over the same period, the incidence of severe ROP (9.6%) and ROP requiring therapy (8.4%) were significantly lower than those reported in other studies (22.5–57.9%) (50-54). In another retrospective case control study of preterm infants born at ≤32 weeks GA on mechanical ventilation, SpO2 histogram was used as one indicator for ventilator adjustments and extubation in the study group. Although there was no significant difference in the duration of oxygen therapy, or the incidence of BPD, ROP and severe brain injury when compared to infants who received routine ventilatory management, rate of failed extubation (6.9% vs. 26.9%, P<0.05) was significantly less and the duration of mechanical ventilation was significantly shorter (median 29 vs. 70 h, P<0.05) in the study group (55).
Oxygenation management for the prevention and treatment of BPD is another area of controversy. Although there was no significant difference in the incidence of physiologic BPD, the NeOProM analysis did show a decreased rate of oxygen dependency in the lower SpO2 group. It is generally accepted that hyperoxia, with SpO2 ≥95% should be avoided in the early postnatal life of extremely premature infants. The Chinese expert consensus on clinical management of premature infants with BPD therefore recommended to initiate resuscitation at 30% FiO2 for infants born at <32 weeks’ GA and maintain SpO2 at 90–94% until 32 weeks corrected age (56). Consistent with the US Pediatric Pulmonary Hypertension Network (PPHNet) recommendation, this Chinese expert consensus also recommended the use of supplemental oxygen with a goal of maintaining SpO2 between 92–95% to avoid episodic or sustained hypoxemia in patients with established BPD (57).
About 1/3 of infants with BPD need to be discharged on oxygen. A study of 1,039 pairs of propensity score matched BPD patients in the US Neonatal Research Network (NRN) demonstrated better growth in those infants discharged on oxygen (58). The ideal target SpO2 during home oxygen therapy (HOT) and the best way of weaning are unknown. However, consistent with most international recommendations, the Chinese expert consensus recommended to keep SpO2 >92% during HOT.
Monitoring of oxygenation
Ideally, titration of supplemental oxygen is best achieved by continuous monitoring of tissue and cellular oxygen delivery. Unfortunately, such monitoring is difficult to achieve in the daily clinical practice. Currently, partial oxygen pressure in arterial blood (PaO2) measurement by arterial blood gas (ABG) and oxygen saturation monitoring through continuous pulse oximeter measurements are the most commonly used methods to monitor oxygenation in the NICUs in China. However, even with careful monitoring, oxygen saturation and PaO2 may fluctuate outside specified ranges, particularly in neonates with cardiopulmonary disease. In recent years, new techniques in oxygenation monitoring, such as transcutaneous PO2 (tcPO2) monitoring, near infrared spectroscopy (NIRS) and lung ultrasound have become available in some of the NICUs, although data to support the utility of these techniques are still limited.
PaO2 monitoring
The “gold standard” of oxygenation monitoring has been the ABG measurement of PaO2. However, this requires repeated blood sampling and cannot provide continuous monitoring. Repeat blood sampling through either arterial puncture or indwelling arterial catheters can cause pain, increase risk of infection, result in desaturations from irritation and/or may affect cerebral blood flow in ELBW.
SpO2 monitoring
With the advantage of being noninvasive and easy to operate, SpO2 monitoring has become the main state of oxygenation monitoring in the NICUs around the world, especially in preterm infants. Major limitations of this monitoring method include moving artifacts, measuring accuracy affected by tissue perfusion, acidosis and blood transfusion with increased hemoglobin A. In addition, it cannot reflect hyperoxia when PaO2 is high (>50 mmHg). Although 90–94% SpO2 is the widely accepted target range for preterm infants on oxygen therapy, as Schmidt pointed out, the local policy of the ideal SpO2 target range should be informed by local patients’ risks for the outcomes. For example, in a unit with high mortality and incidence of NEC, but relatively low rates of ROP, adapting the higher oxygen target range might be more appropriate. However, if the major concern in a unit is severe BPD and ROP, tighter control of SpO2 could be more beneficial (59). In addition to the ideal SpO2 target range, local resource should also be taken into consideration when choosing the alarm limit settings to ensure timely responses to critical alarms while minimizing staff “alarm fatigue”. McClure et al. reported over 3 million alarms over 1 year in their NICU, most of which were due to low or high oxygen saturation alarms. This translated into an average of 250 alarms per infant per day and 30 alarms per nurse per hour. They also reported that longer SpO2 averaging times masked the number and severity of aberrant oxygenation events. However, incorporating an alarm delay with shorter SpO2 averaging times could reduce alarm number and duration while allowing for more accurate assessment of oxygenation (60).
In the Chinese NICUs with high nursing to patient ratio, it is unrealistic to expect nurses to respond to all SpO2 alarms and accurately titrate oxygen on multiple preterm infants while performing their many other patient care responsibilities. Automated oxygen adjustment systems have therefore been developed with some data in the neonatal population published in the recent years (61). Such system is available in some ventilators in China. However, the utility of this technique and data from the Chinese neonates are scarce, partly due to the limited availability of this technology in the Chinese NICUs.
Similarly, the utility of oxygen saturation histograms in the NICU are being explored both in China and internationally. Using SpO2 histograms, Miller-Barmak et al. demonstrated that VLBW infants on noninvasive respiratory support had decreased oxygen instability and higher oxygenation on prone positioning (62). In a large regional NICU in Alabama, US, incorporating SpO2 histogram monitoring into clinical care of extremely premature infants born at <29 weeks’ gestation resulted in improved time at goal saturations and a reduction in death or sever ROP (63). In the same year, a review in Chinese of the utility of oxygen saturation histogram in guiding respiratory support in preterm infants was published (64), and Huang et al. also published a retrospective study reporting their use of oxygen saturation histogram monitoring in mechanical ventilated preterm infants (55). Unfortunately, only few brands of monitors can provide oxygen saturation histogram, which significantly limited its use in the care of preterm infants in China.
tcPO2 monitoring
Given the difficulty of ABG monitoring, transcutaneous PCO2 monitoring, using conventional electrochemical techniques, has been increasing utilized in the NICUs in China. This noninvasive technique is relatively simple to use, has been shown to have good correlation with blood PCO2 level, and provides a means of continuous monitoring. However, the utility of tcPO2 monitoring is much more controversial. Multiple studies have examined the application of tcPO2 monitoring in the Chinese NICUs. Studies examining the accuracy of tcPO2 monitoring are conflicting: Liu and He reported good correlation whereas Ren et al. and Xu et al. reported poor correlation between tcPO2 and PaO2 (65-67). One study of 60 critically ill neonates born at 29–37 weeks’ GA found that placing the electoral probe on the thigh resulted in best correlation with blood PaO2 and PCO2 levels and least likely to fall off, as compared to placing the probe on the chest and abdomen (68). In another study, Li et al. examined the effect of electrode temperature on tcPCO2 and tcPO2 measurement in 45 VLBW infants. Using one probe at 42 °C as control, the authors found that there were no significant difference in tcPCO2 levels at any probe temperature, whereas tcPO2 were all significantly lower when measure at lower probe temperature of 38–41 °C. Since there were still positive correlations between these measurements and those measured at 42 °C or PaO2, the authors concluded that the values measured at lower temperature may be used to monitor the trend of PaO2 changes (69).
NIRS monitoring
Using a technique similar to pulse oximetry, NIRS calculates tissue oxygenation status based on the different degrees of absorption and attenuation of near-infrared light by different hemoglobin in the tissue, and therefore can provide information of regional tissue oxygenation (rSO2) status. It allows for continuous noninvasive monitoring of tissue oxygenation of multiple organ systems (brain, intestine, kidneys, muscles and lungs) in both extremely preterm infants and other critically ill newborns. Multiple studies have studied rSO2 in multiple organs, particularly cerebral (CrSO2), renal (RrSO2) and splanchnic beds (SrSO2) in both normal full-term neonates (70-72) and preterm infants (73-75). Similar studies in healthy Chinese neonates or during different disease states have emerged in recent years and main results are summarized in Figure 1 (76-81).
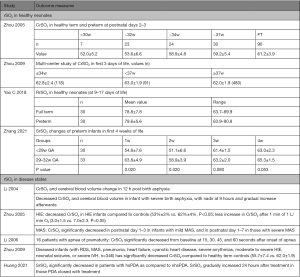
Although NIRS had been introduced in Chinese NICUs since 1990s, it had not been widely used in the Chinese NICUs. Besides the high cost of the device, other reasons for its limited use include large variation in the measured levels even in the same patient, and threshold for intervention is unknown. Therefore, this technique is mostly used in research settings.
Summary
Evidence-guided neonatal oxygenation management in China has evolved to the level of western countries with more advanced neonatal intensive care in many areas such as neonatal resuscitation and preterm oxygenation management. However, significant variations of care levels exist and many factors including inadequate personnel, equipment shortage and lack of high-quality research to guide clinical practice hinders the further development of neonatal oxygenation management in China. Based on our review, the following suggestions, targeting specific problems in China, might be helpful in improving neonatal oxygenation management in the Chinese population:
- Standardize the use of oxygen blender and pulse oximeter in both the delivery room and the NICUs;
- Setting unit SpO2 target in extremely preterm infants and establishing unit protocol to maintain the set target;
- Early application of adequate end expiratory pressure, either via CPAP or PEEP, in extremely premature infants and other infants with respiratory distress;
- Proper use of available respiratory support devices, both invasive and non-invasive, to provide flow and positive pressure support, and avoiding unblended oxyhood or free flow oxygen use;
- Enhanced training of neonatal care team in neonatal oxygenation management to improve understanding of pathophysiology, current evidence, proper use of available equipment, and multidisciplinary team collaboration;
- Increase availability of iNO in Chinese NICUs;
- Heightened regulation and training for neonatal ECMO programs.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ola Didrik Saugstad) for the series “Oxygen in the Newborn” published in Pediatric Medicine. The article has undergone external peer review.
Peer Review File: https://pm.amegroups.com/article/view/10.21037/pm-22-52/prf
Reporting Checklist: The authors have completed the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-22-52/rc).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-22-52/coif). The series “Oxygen in the Newborn” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
※Special series on Oxygen in the Newborn.
References
- WHO. Neonatal mortality. Available online: https://www.who.int/data/gho/data/countries/country-details/GHO/china?countryProfileId=adf73789-9c42-4bc5-a39b-b4d7ba337beb
- Cao Y, Jiang S, Sun J, et al. Assessment of Neonatal Intensive Care Unit Practices, Morbidity, and Mortality Among Very Preterm Infants in China. JAMA Netw Open 2021;4:e2118904. [Crossref] [PubMed]
- Zhu X. Neonatal resuscitation. World Health Forum 1993;14:289-90.
- Xu T, Wang H, Gong L, et al. The impact of an intervention package promoting effective neonatal resuscitation training in rural China. Resuscitation 2014;85:253-9. [Crossref] [PubMed]
- Xu T, Wang HS, Ye HM, et al. Impact of a nationwide training program for neonatal resuscitation in China. Chin Med J (Engl) 2012;125:1448-56.
- Xu T, Wang H, Gong L, et al. Midterm evaluation of the national neonatal resuscitation program in China. Chin J Perinat Med 2009;12:409-12.
- Xu T, Yue Q, Wang H, et al. Evaluation on the second phase of Neonatal Resuscitation Program in China. Chin J Perinat Med 2017;20:346-51.
- Sotiropoulos JX, Kapadia V, Vento M, et al. Oxygen for the delivery room respiratory support of moderate-to-late preterm infants. An international survey of clinical practice from 21 countries. Acta Paediatr 2021;110:3261-8. [Crossref] [PubMed]
- Kattwinkel J. Neonatal Resuscitation. 6th edition. Beijing: People's Medical Publishing House; 2012.
- China Neonatal Resuscitation Program Task Force NRS, Society of Perinatal Medicine, Chinese Medical Association. China neonatal resuscitation guideline (revised in 2021) Chin J Perinat Med (Chinese) 2022;25:4-12.
- Welsford M, Nishiyama C, Shortt C, et al. Room Air for Initiating Term Newborn Resuscitation: A Systematic Review With Meta-analysis. Pediatrics 2019;143:e20181825. [Crossref] [PubMed]
- American Academy of Pediatrics AHA. 8th ed. Textbook of Neonatal Resuscitation (NRP). Amedican Academy of Pediatrics; 2021.
- Aziz K, Lee HC, Escobedo MB, et al. Part 5: Neonatal Resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020;142:S524-50. [Crossref] [PubMed]
- Mariani G, Dik PB, Ezquer A, et al. Pre-ductal and post-ductal O2 saturation in healthy term neonates after birth. J Pediatr 2007;150:418-21. [Crossref] [PubMed]
- Wang H, Yang Y, Lu C, et al. The reference ranges of oxygen saturation and heart rate in healthy infants during the first ten minutes after birth. Journal of Clinical Pediatrics 2014;32:206-9.
- Xia C, Liu Q, Liu Y, et al. The reference ranges of oxygen saturation and heart rate in healthy infants during the first ten minutes after birth. J Clin Pediatr (Chinese) 2019;37:485-8.
- Escrig R, Arruza L, Izquierdo I, et al. Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: a prospective, randomized trial. Pediatrics 2008;121:875-81. [Crossref] [PubMed]
- Vento M, Moro M, Escrig R, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 2009;124:e439-49. [Crossref] [PubMed]
- Kapadia VS, Chalak LF, Sparks JE, et al. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics 2013;132:e1488-96. [Crossref] [PubMed]
- Tataranno ML, Oei JL, Perrone S, et al. Resuscitating preterm infants with 100% oxygen is associated with higher oxidative stress than room air. Acta Paediatr 2015;104:759-65. [Crossref] [PubMed]
- Dawson JA, Kamlin CO, Wong C, et al. Oxygen saturation and heart rate during delivery room resuscitation of infants <30 weeks' gestation with air or 100% oxygen. Arch Dis Child Fetal Neonatal Ed 2009;94:F87-91. [Crossref] [PubMed]
- Rabi Y, Lodha A, Soraisham A, et al. Outcomes of preterm infants following the introduction of room air resuscitation. Resuscitation 2015;96:252-9. [Crossref] [PubMed]
- Oei JL, Saugstad OD, Lui K, et al. Targeted Oxygen in the Resuscitation of Preterm Infants, a Randomized Clinical Trial. Pediatrics 2017;139:e20161452. [Crossref] [PubMed]
- Editorial Board. Chinese Journal of Pediatrics; The Subspecialty Group of Neonatology, the Society of Pediatrics, Chinese Medical Association; Editorial Board Chinese Journal of Pediatrics The Subspecialty Group of Neonatology the Society of Pediatrics Chinese Medical Association. Routine of mechanical ventilation in neonates. Zhonghua Er Ke Za Zhi 2015;53:327-30.
- Song C, He Y. Oxygen concentration analyzer combines with blood gas analysis to lead the choice of oxygen flow with oxygen hood for the newborn. Anhui Medical Journal 2012;33:1137-8.
- Xu Y, Cheng H. Study on the clinical effect of nasal high-flow catheter wet oxygen therapy in treatment of neonatal pneumonia complicated with respiratory failure. Maternal and Child Health Care of China 2017;32:3077-80.
- Eghbalian F. A comparison of supine and prone positioning on improves arterial oxygenation in premature neonates. J Neonatal Perinatal Med 2014;7:273-7. [Crossref] [PubMed]
- Tang Y, Lu Z, Ju H. Effect of prone positioning in neonates with acute lung injury and acute respiratory distress syndrome. Electronic Journal of Practical Gynecologic Endocrinology 2020;7:192-3.
- Zhang W, Wu YE, Yang XY, et al. Oral drugs used to treat persistent pulmonary hypertension of the newborn. Expert Rev Clin Pharmacol 2020;13:1295-308. [Crossref] [PubMed]
- He Z, Zhu S, Zhou K, et al. Sildenafil for pulmonary hypertension in neonates: An updated systematic review and meta-analysis. Pediatr Pulmonol 2021;56:2399-412. [Crossref] [PubMed]
- Yan GF, Cai XD, Zhou CB, et al. Multicenter investigation of extracorporeal membrane oxygenation application in pediatric intensive care unit in China. Zhonghua Er Ke Za Zhi 2018;56:929-32. [Crossref] [PubMed]
- Zhou C, Guo Y, Hu Y. Current development of pediatric extracorporeal membrane oxygenation technique in China. Chinese Journal of New Clinical Medicine 2021;14:427-31.
- Askie LM, Henderson-Smart DJ, Ko H. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants. Cochrane Database Syst Rev 2009;2009:CD001077. [Crossref] [PubMed]
- Askie LM, Brocklehurst P, Darlow BA, et al. NeOProM: Neonatal Oxygenation Prospective Meta-analysis Collaboration study protocol. BMC Pediatr 2011;11:6. [Crossref] [PubMed]
- Cummings JJ, Polin RACommittee on Fetus and Newborn. Oxygen Targeting in Extremely Low Birth Weight Infants. Pediatrics 2016;138:e20161576. [Crossref] [PubMed]
- Sweet DG, Carnielli V, Greisen G, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2016 Update. Neonatology 2017;111:107-25. [Crossref] [PubMed]
- Neonatologist Branch of Chinese Medical Doctor A. the Editorial B, Chinese Journal of P. Guideline for the clinical application of nasal intermittent positive pressure ventilation in premature infants (version 2019). Zhonghua Er Ke Za Zhi 2019;57:248-51. [Crossref] [PubMed]
- Chen Y, Xun D, Wang YC, et al. Incidence and risk factors of retinopathy of prematurity in two neonatal intensive care units in North and South China. Chin Med J (Engl) 2015;128:914-8. [Crossref] [PubMed]
- Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet 2013;382:1445-57. [Crossref] [PubMed]
- Higgins RD. Oxygen Saturation and Retinopathy of Prematurity. Clin Perinatol 2019;46:593-9. [Crossref] [PubMed]
- Zhu Y, Jiang S, Wang X, et al. Incidence of retinopathy of prematurity among infants less than 34 weeks' gestation before discharge in 25 NICUs in China: A cross-sectional survey. Chinese Journal of Evidence-Based Pediatrics 2020;15:45-9.
- Li L, Gao Y, Chen W, et al. Screening for retinopathy of prematurity in North China. BMC Ophthalmol 2022;22:251. [Crossref] [PubMed]
- Neonatologists Branch of CMDA. Oxygen therapy in preterm infants for the prevention and treatment of retinopathy of prematurity. Journal of Developmental Medicine 2016;4:196-8.
- Mao Y, Liu T, Wang J, et al. Analysis of retinopathy of prematurity with birth weight between 2kg and 2.5kg in GuangZhou area. Chinese Journal of Strabismus & Pediatric Ophthalmology 2018;26:14-7.
- Yang Q, Zhou X, Ni Y, et al. Optimised retinopathy of prematurity screening guideline in China based on a 5-year cohort study. Br J Ophthalmol 2021;105:819-23. [Crossref] [PubMed]
- Tiryaki Demir S, Karapapak M, Uslu HS, et al. Retinopathy screening results of late-preterm infants born at 32-35 weeks of gestational age. Graefes Arch Clin Exp Ophthalmol 2019;257:1325-9. [Crossref] [PubMed]
- Das A, Mhanna M, Sears J, et al. Effect of fluctuation of oxygenation and time spent in the target range on retinopathy of prematurity in extremely low birth weight infants. J Neonatal Perinatal Med 2018;11:257-63. [Crossref] [PubMed]
- Gantz MG, Carlo WA, Finer NN, et al. Achieved oxygen saturations and retinopathy of prematurity in extreme preterms. Arch Dis Child Fetal Neonatal Ed 2020;105:138-44. [Crossref] [PubMed]
- Guo D, Zhang F, Tong G, et al. Analysis of Oxygen Therapy and Related Diseases in Severe Active Retinopathy of Prematurity. Chinese Journal of Optometry Ophthalmology and Visual Science 2020;22:769-74.
- Mao J, Yu X, Shen L, et al. Risk factors of retinopathy of prematurity in extremely low birth weight infants by strictly controlling oxygen inhalation after birth. Chinese Journal of Ophthalmology 2019;55:280-8. [Crossref] [PubMed]
- Li QP, Wang ZH, Li YY, et al. Retinopathy of prematurity screening in 2185 premature infants. Zhonghua Yan Ke Za Zhi 2012;48:903-7.
- Shenzhen Retinopathy of Prematurity (ROP) Collaboration Group. The incidence of retinopathy of prem aturity in Shenzhen during the past ten years. Chinese Journal of Ocular Fundus Diseases 2014;30:12-6.
- IZhou Y. Multicenter survey on the clinical features and fundus lesions of retinopathy in premature infants in mainland China. Chinese Journal of Evidence-Based Pediatrics 2015;10:161-5.
- Wang J, Xiang D, Chen F, et al. Retinopathy of prematurity in 857 very low birth weight infants. Chinese Journal of Practical Ophthalmology 2015;33:21-4.
- Huang D, Huang Y, Jin H, et al. Utility of oxygen saturation histogram in mechanical ventilation of preterm infants ≤32 weeks’ gestation. Chinese Journal of Neonatology 2020;35:439-42.
- Subspecialty Group of Neonatology. the Society of Pediatrics, Chinese Medical Association; Editorial Board, Chinese Journal of Pediatrics. Expert consensus on clinical management of premature infants with brochopulmonary dysplasia. Zhonghua Er Ke Za Zhi 2020;58:358-65. [Crossref] [PubMed]
- Krishnan U, Feinstein JA, Adatia I, et al. Evaluation and Management of Pulmonary Hypertension in Children with Bronchopulmonary Dysplasia. J Pediatr 2017;188:24-34.e1. [Crossref] [PubMed]
- DeMauro SB, Jensen EA, Bann CM, et al. Home Oxygen and 2-Year Outcomes of Preterm Infants With Bronchopulmonary Dysplasia. Pediatrics 2019;143:e20182956. [Crossref] [PubMed]
- Schmidt B, Whyte RK. Oxygen saturation target ranges and alarm settings in the NICU: What have we learnt from the neonatal oxygenation prospective meta-analysis (NeOProM)? Semin Fetal Neonatal Med 2020;25:101080. [Crossref] [PubMed]
- McClure C, Jang SY, Fairchild K. Alarms, oxygen saturations, and SpO2 averaging time in the NICU. J Neonatal Perinatal Med 2016;9:357-62. [Crossref] [PubMed]
- Claure N, Bancalari E. Targeting Arterial Oxygen Saturation by Closed-Loop Control of Inspired Oxygen in Preterm Infants. Clin Perinatol 2019;46:567-77. [Crossref] [PubMed]
- Miller-Barmak A, Riskin A, Hochwald O, et al. Oxygenation Instability Assessed by Oxygen Saturation Histograms during Supine vs Prone Position in Very Low Birthweight Infants Receiving Noninvasive Respiratory Support. J Pediatr 2020;226:123-8. [Crossref] [PubMed]
- Gentle S, El-Ferzli G, Winter L, et al. Oxygen saturation histogram monitoring to reduce death or retinopathy of prematurity: a quality improvement initiative. J Perinatol 2020;40:163-9. [Crossref] [PubMed]
- Zhu W, Huang Y, Zhuang D, et al. Research advances in oxygen saturation histogram to guide the respiratory support therapy of very preterm infants. Chinese Pediatric Emergency Medicine 2020;27:933-5.
- Liu Y, He S. Transcutaneous CO2 and transcutaneous O2 monitoring in neonatal intensive care unit. Chinese Journal of Neonatology 2009;24:15-7.
- Ren Y, Yang C, Chen H, et al. A study on the value of transcutaneous carbon dioxide/oxygen monitoring in NICU. Chinese Journal of Neonatology 2015;30:98-103.
- Xu J, Wang R, Wu L, et al. Transcutaneous CO2 and transcutaneous O2 monitoring in the evaluation of neonatal respiratory failure. Chinese Journal of Neonatology 2018;33:437-41.
- Li P, Qian W. Clinical observation on monitoring different body parts of neonatal intensive care unit critically ill newborns by percutaneous oxygen partial pressure and carbon dioxide partial pressure. Chinese Journal of Practical Nursing 2019;35:1084-7.
- Li BH, Zhao CL, Cao SL, et al. Effect of electrode temperature on measurements of transcutaneous carbon dioxide partial pressure and oxygen partial pressure in very low birth weight infants. Zhongguo Dang Dai Er Ke Za Zhi 2021;23:809-13. [Crossref] [PubMed]
- Urlesberger B, Kratky E, Rehak T, et al. Regional oxygen saturation of the brain during birth transition of term infants: comparison between elective cesarean and vaginal deliveries. J Pediatr 2011;159:404-8. [Crossref] [PubMed]
- Baik N, Urlesberger B, Schwaberger B, et al. Reference Ranges for Cerebral Tissue Oxygen Saturation Index in Term Neonates during Immediate Neonatal Transition after Birth. Neonatology 2015;108:283-6. [Crossref] [PubMed]
- Bailey SM, Hendricks-Munoz KD, Mally P. Cerebral, renal, and splanchnic tissue oxygen saturation values in healthy term newborns. Am J Perinatol 2014;31:339-44. [Crossref] [PubMed]
- Roche-Labarbe N, Carp SA, Surova A, et al. Noninvasive optical measures of CBV, StO(2), CBF index, and rCMRO(2) in human premature neonates' brains in the first six weeks of life. Hum Brain Mapp 2010;31:341-52. [Crossref] [PubMed]
- McNeill S, Gatenby JC, McElroy S, et al. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J Perinatol 2011;31:51-7. [Crossref] [PubMed]
- Hyttel-Sorensen S, Pellicer A, Alderliesten T, et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ 2015;350:g7635. [Crossref] [PubMed]
- Zhou C, Tang Z, Huang L. Researches on the Cerebral Developments and Impairments of Neonates Using NIRS. Chinese Journal of Medical Instrumentation 2005;11:30e-g.
- Zhou CL, Liu YF, Zhang JJ, et al. Measurement of brain regional oxygen saturation in neonates in China: a multicenter randomized clinical trial. Zhonghua Er Ke Za Zhi 2009;47:517-22.
- Yao C, Xie Y, Ma X, et al. Monitoring of Renal Regional Oxygenation Saturation in Healthy Infants by Near-infrared Spectroscopy. Medical Innovation of China 2018;15:54-7.
- Zhang JH, Guan RL, Pan PP, et al. Changing trend of abdominal regional oxygen saturation in very/extremely low birth weight infants in the early postnatal stage: a prospective study. Zhongguo Dang Dai Er Ke Za Zhi 2021;23:1015-20. [Crossref] [PubMed]
- Li Z, Wen F, Yang J, et al. Regional oxygen saturation and blood volume of cerebrum in apnea of prematurity. Chinese Journal of Clinical Medicine 2006;2:117-8.
- Huang XB, Zhong X, Liu T, et al. Value of near-infrared spectroscopy in monitoring intestinal tissue oxygen saturation in preterm infants with hemodynamically significant patent ductus arteriosus: a prospective research. Zhongguo Dang Dai Er Ke Za Zhi 2021;23:821-7. [Crossref] [PubMed]
Cite this article as: Sun Y, Rong X, Zhang H. Oxygenation in the newborn: a narrative review in Chinese perspective. Pediatr Med 2023;6:39.

