Effects of maternal folic acid supplementation on renal urinary system development in human offspring—a meta-analysis and systemic review
Highlight box
Key findings
• Maternal folic acid (FA) supplementation might be relevant to offspring congenital anomalies of the kidney and urinary tract (CAKUT), especially cystic kidney.
What is known and what is new?
• Previous studies had found that the use of FA during pregnancy indeed has a protective effect against neural tube developmental malformation.
• Maternal FA supplementation may be associated with offspring CAKUT, especially cystic kidney.
What is the implication, and what should change now?
• Prescription of FA supplementation for pregnant women should be carefully censored, especially for pregnant mothers with a family history of kidney disease. The precise-timing and individuated-dose for FA supplementation during pregnancy remains unclear and needs further research.
• The overall limited quality of relevant case-control studies necessitates further high-quality researches.
Introduction
Congenital anomalies of the kidney and urinary tract (CAKUT) refer to a group of malformations. The international occurrence of CAKUT is as high as 6/1,000, accounting for 30–50% of all prenatal diagnostic malformations, which is the main reason for end-stage kidney disease in children (1).
By constructing a variety of knockout animal models, such as zebrafish, toad, and mice models, and screening human pathogenic genes, most scholars currently believe that gene mutations and environmental factors are the two main factors of CAKUT pathogenesis (2). In recent years, adverse environmental factors during pregnancy have gradually attracted the attention of researchers, who believe that adverse intrauterine environmental factors also play an essential role in the pathogenesis of CAKUT. On the one hand, large population retrospective studies discovered some adverse intrauterine factors correlated with CAKUT, such as a low-protein diet, gestational diabetes, drinking, smoking, and folate deficiency. On the other hand, by constructing mouse models of a low-protein diet during pregnancy, intrauterine hypoxia, and gestational diabetes mellitus to simulate the adverse factors of intrauterine growth retardation (IUGR) (3), hypoxia (4), and high sugar (5), it was confirmed that the above factors could increase the incidence of CAKUT in mice.
A recent study (6) compared the differences in intrauterine environmental factors in 562 patients with CAKUT and 2,139 normal control participants and proposed that in vitro fertilization and gestational diabetes may be relevant to the occurrence of CAKUT, while maternal usage of folic acid (FA) increases the risk of CAKUT. The Canfield MA (7) and Godwin KA (8) studies also suggested that FA use is associated with CAKUT. Moreover, some studies (9) have found that the use of FA during pregnancy does not adversely affect children or cause renal urinary system malformations but indeed has a protective effect against neural tube developmental malformations. In summary, the current research on the effect of FA supplementation on the development of the renal urinary system is controversial, and there is a lack of reports on relevant animal models.
Therefore, a meta-analysis review and analysis of the relevance of maternal FA supplementation and renal urinary system structural abnormalities in human offspring was proposed. We present this article in accordance with the PRISMA reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-23-11/rc) (10).
Methods
This systematic review protocol was registered with the online Prospero database (CRD42023397185) (11).
Search strategy
We searched the PubMed, Web of Science, CINAHL, Cochrane, EMBASE, MEDLINE, and Scopus databases. The search terms included three groups: (I) pregnancy or pregnant* or lateral or prenatal or maternal or mother*; (II) folate or felsite or folacin or “folic acid, *” or “vitamin B9” or “vitamin M, *” or “B9, vitamin” or “pteroylglutamic acid”; and (III) kidney or nephron* or renal or urinary or uret*.
Inclusion criteria
Types of participants
This meta-analysis review only included human research, and all female participants had to be pregnant (at any stage of gestation or in the periconceptional period) and successfully deliver the baby during the time of the study.
Types of studies
This meta-analysis review involved experimental studies, including pre–post studies, pseudo-randomized controlled trials (RCTs), and experimental RCTs, and observational studies, including case-control studies, cross-sectional studies, and prospective and retrospective cohort studies.
Types of interventions
The intervention in this meta-analysis was maternal FA supplementation during pregnancy (at any stage of gestation or in the periconceptional period).
Types of outcome measures
Studies that included a measure of offspring kidney structure as a primary or secondary outcome were considered for inclusion.
Study selection
All identified articles were retrieved and exported to EndNote and screened following four steps: (I) titles, abstracts, descriptions, and MeSH headings were used to assess study inclusion; (II) full texts for the literature that satisfied the inclusion criteria from step 1 were retrieved; (III) the final full papers were retrieved for review and data extraction; and (IV) all steps were completed independently by two reviewers, and when there was disagreement at any step, a final decision was independently made by a third reviewer.
Study quality
All included studies were assessed for methodological quality using the American Dietetic Association Quality Criteria Checklist (12), and the overall quality of the final studies was rated as positive, neutral, or negative by two independent reviewers, while a third reviewer was consulted when needed. No studies were excluded based on quality ratings.
Data extraction and synthesis
One reviewer carried out data extraction and was cross-checked by a second reviewer independently, including data on the study design, intervention characteristics, participant information, and data related to review outcomes. Meta-analysis of data was not expected due to folate deficiency in pregnant women, heterogeneity in folate supplementation, and measures of renal urinary system outcomes. Thus, the influence of maternal FA supplementation on offspring renal urinary system structure is described in a narrative review.
Statistical analysis
The meta-analysis used a random effects model to obtain the total odds ratio (OR) for each outcome through Review Manager (5.4.1). All statistical analyses were performed using STATA. Unless otherwise stated, P<0.05 was considered statistically significant.
Results
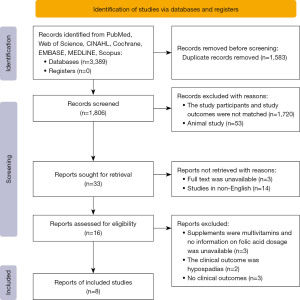
Study selection
The initial literature screening identified 1,806 articles after removing duplicate papers (Figure 1), and 1,773 articles were excluded following the title and abstract review. Despite online interlibrary searches, the full texts of 3 studies were unavailable, and 14 studies were not published in English. Ultimately, 16 full-text papers were retrieved. The reasons for the removal of full-text articles were as follows: the outcomes were not related to offspring renal outcomes (n=2); detailed clinical kidney outcomes were not reported (n=3); and the supplements were multivitamins for which no detailed information on FA dosage was available (n=3). The remaining 8 articles were included in this current meta-analysis and focused on maternal FA supplementation and offspring renal urinary system outcomes.
Study characteristics
The 8 included articles were published from 1998 to 2020. In the included literature (Table 1), RCTs (n=1), controlled cohort trials (n=1), case-control studies (n=3), a prospective study (n=1) and descriptive studies (n=2) illustrated the association between maternal FA supplementation and offspring kidney malformations. Only 3 articles did not have the detailed dose of FA supplementation during pregnancy: the study by Groen In 't Woud et al. (6) used only the questionnaire method to determine whether the pregnant mothers used folic acid products during pregnancy, and the dose was not described in detail; the study by Canfield (7) and Botto (17) was performed after the implementation of the FA-fortification policy, and the trend of renal development malformations in offspring before and after the FA-fortification intervention were compared; and the study by Botto evaluated the specific strengthening policy (17).
Table 1
| References | Setting | Aims | Design | Sample size | Years | Details of intervention | Data acquisition |
|---|---|---|---|---|---|---|---|
| Gildestad 2020 (13) | Medical Birth Registry of Norway (MBRN) | Compared the risks of organ-specific major birth defects in live- and stillborn infants of mothers who did or did not use folic acid and/or multivitamin supplements | Prospective study | 894,927 live and stillborn infants | 1999 to 2013 | Folic acid tablets available over the counter in Norway during the study period contained 0.4 mg of folic acid. The maximum limit of folic acid in multivitamins was 0.2 mg | Information on supplement use is collected during pregnancy and transferred to the birth notification form |
| Groen In 't Woud 2016 (6) | AGORA (Aetiologic research into Genetic and Occupational/environmental Risk factors for Anomalies in children) data- and biobank | Studied potential maternal risk factors in individual phenotypes within the congenital anomalies of the kidney and urinary tract (CAKUT) spectrum | Case-control study | 562 children with CAKUT; 2,139 healthy controls | January 1990 to March 2011 | Mothers were considered to have used folic acid when they reported use of folic acid supplements only, folic acid-containing multivitamins, or a combination of folic acid and multivitamin supplements from 1 month before conception through the eighth week after conception | Questionnaire data |
| Bower 2006 (9) | Non-neural birth defects in Western Australia | Investigate whether maternal periconceptional folate intake is associated with a reduction in selected non-neural birth defects in Western Australia | Case-control study | Case 522; Control 578 | January 1997 to December 1999 | Women consuming an average of 0.2 mg or more daily from supplements for each of four time periods the month before pregnancy and each of the first three months of pregnancy were considered to be adequately supplemented and were compared with women taking less than 0.2 mg a day | All eligible case and control women were invited by mail to complete a pregnancy/demographic questionnaire and a semiquantitative food frequency dietary questionnaire |
| Canfield 2005 (7) | National Birth Defects Prevention Network (NBDPN) | Examined whether folic acid fortification might have decreased the prevalence of other specific birth defects | Descriptive study | Using population-based data from 23 state member programs of the NBDPN | 1995 to 2000 | Optional fortification of the grain supply was initiated as early as late 1996, by law, fortification of all enriched grain products was to be completed by January 1, 1998 | Focused on two delivery time periods, 1995–1996 and 1999–2000 |
| Czeizel 2004 (14) | Hungarian Periconceptional Service | The 1984–1991 Hungarian randomized controlled trial of periconceptional multivitamin supplementation (PMVS) containing folic acid showed a significant reduction in the first occurrence of neural tube defects, and of urinary tract and cardiovascular abnormalities, but no reduction in orofacial clefts. A controlled cohort trial was designed to confirm or deny these results | Controlled cohort trial | Supplemented cohort 3,056; Unsupplemented cohort 3,056 | May 1993 to April 1996 | Supplemented women were recruited from the HPS using the same multivitamin as the Hungarian randomized controlled trials (RCTs). PMVS containing 0.8 mg folic acid. Unsupplemented pregnant women were recruited in the standard regional antenatal care clinics and were matched to each supplemented pregnant woman on the basis of age, socioeconomic status, place of residence, and year of pregnancy | Both supplemented and unsupplemented cohort women were asked to bring their completed pregnancy outcome certificate personally or send it by mail to the appropriate center of the HPS or cohort-controlled trial after the completion of their pregnancy |
| De Walle 2003 (15) | European Surveillance of Congenital Anomalies (EUROCAT) northern Netherlands registry | Investigated the possible preventive effect of folic acid in five selected groups of malformations | Case-control study | 267,937 live and stillborn infants; 2,751 cases were defined as infants with folic acid (FA) sensitive defects; 3,647 controls were all children and fetuses with anomalies other than the sensitive anomalies | 1981 to 1998 | Folic acid intake was defined as intake of 0.4 mg of folic acid daily starting before conception until 8 weeks after conception. If mothers took some folic acid but not during the entire period the infants were excluded from the analyses | Information on FA use was collected via the physician, pharmacist and mother |
| Czeizel 1998 (16) | Hungarian Periconceptional Service (HPS) | A summary about the final results of the Hungarian double-blind placebo controlled randomized trial of periconceptional folic acid containing multivitamin and trace element supplementation | Randomized clinical trials | 3,953 multivitamins; 3,952 trace element | 1984 to 1991 | Supplemented women were recruited from the HPS using the same multivitamin as the Hungarian RCT. The Hungarian double-blind randomized trial of PMVS (0.8 mg) and placebo like trace element supplementation was part of the periconceptional care | Women were asked to bring their completed pregnancy outcome certificate personally or send it by mail to the appropriate center of the HPS |
| Botto 2006 (17) | 15 registries that are members of the International Clearinghouse for Birth Defects Monitoring Systems (recently renamed International Clearinghouse for Birth Defects Surveillance and Research) | Whether supplementation recommendations alone, without fortification, are effective in reducing the population-wide rates of neural tube defects, and whether such policies can reduce the occurrence of other birth defects | Descriptive study | 1.5 million births yearly (1,000,000 from Europe, 370,000 from Canada and the United States, and 87,000 from Australia) | 1980 to 2003 | Recommend the use of daily supplements 1 from before conception varied by countries, regions | Gathered information on folic acid policies from interviews of the Program Director of the registry, publications listed on MEDLINE, reports of workshops and committees, and documents issued by governmental agencies and professional bodies |
Maternal FA supplementation and renal urinary system defects
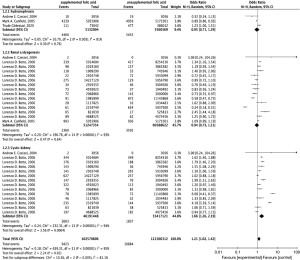
In this study, 8 original articles were included to explore the association between maternal FA supplementation and offspring renal urinary system development malformations, and a total of 48,796,782 participants were included in the FA-supplemented group and 58,394,898 participants were included in the FA-unsupplemented group. The forest plot (Figure 2) shows that maternal FA supplementation was related to an increased risk of offspring CAKUT (OR =1.14; 95% CI, 1.02–1.27; P=0.02). However, there was significant heterogeneity in the results of all studies (I2=88%).

CAKUT clinical phenotypes are abundant, and different environmental factors may inconsistently increase the risk of CAKUT with special phenotypes. Thus, subgroup analysis of patients with different types of CAKUT, including hydronephrosis, renal a/dysgenesis, and cystic kidney, was performed in this study. The results (Figure 3) demonstrated that maternal FA supplementation contributed to a significantly increased risk of offspring cystic kidney (OR =1.66; 95% CI, 1.26–2.19; P=0.0004). However, the risk of offspring hydronephrosis (OR =0.95; 95% CI, 0.71–1.29; P=0.76) and the effect of renal a/dysgenesis (OR =0.94; 95% CI, 0.73–1.21; P=0.64) showed non-statistical significance. At the same time, after CAKUT grouping, the heterogeneity of each group was still high (I2=81.1%).
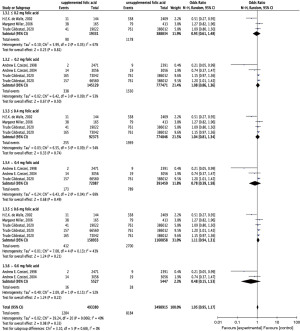
Different doses of FA supplementation can be related to the incidence of CAKUT, so this study additionally defined the FA supplementation dose in the subgroup analysis, including five studies with exact FA supplementation measurements, and grouped participants based on FA supplementation (doses of 0.2 mg per day, 0.4 mg per day, and 0.6 mg per day). Heterogeneity was appreciably decreased among the subgroups (I2=0) (Figure 4). When FA supplementation was ≤0.2 mg/day for the duration of pregnancy, the CAKUT incidence in offspring was decreased (OR =0.95; 95% CI, 0.61–1.48; P=0.81). When FA supplementation was >0.2 mg/day during pregnancy, the incidence of CAKUT increased in offspring (OR =1.08; 95% CI, 0.86–1.36; P=0.50). When FA supplementation was ≤0.4 mg/day during pregnancy, the prevalence of CAKUT increased (OR =1.04; 95% CI, 0.81–1.34), even though there was no statistically significant difference (P=0.74). When maternal FA supplementation was >0.4 mg/day throughout pregnancy, the prevalence of CAKUT in offspring decreased (OR =0.78; 95% CI, 0.39–1.58; P=0.49). When FA supplementation was ≤0.6 mg/day throughout pregnancy, the prevalence of CAKUT in offspring was increased (OR =1.11; 95% CI, 0.94–1.31; P=0.21). At >0.6 mg/day of FA supplementation during pregnancy, the prevalence of CAKUT in offspring decreased (OR =0.48; 95% CI, 0.15–1.53; P=0.22).
Discussion
This meta-analysis clarified that maternal FA supplementation might increase the incidence of renal urinary malformations in offspring, mainly the incidence of cystic kidney. However, different doses of folic acid supplementation did not suggest a significant difference. To date, this is the first systematic review and meta-analysis to focus on the association between maternal FA supplementation and offspring renal urinary malformations.
Maternal FA supplementation has an obvious protective effect on decreasing the risk of neural tube defects (NTDs) in offspring. However, whether maternal folic acid supplementation has an equal protective impact against birth defects in other systems, such as the renal urinary system, remains inconclusive. Among these birth defects, CAKUT is a series of congenital defects with a complicated etiology. In recent years, with the investigation of massive sample populations, a few high-hazard maternal pathogenic factors associated with CAKUT in offspring were discovered. Bower (9) discovered that mothers without FA supplementation had a high risk for CAKUT in their offspring. Czeizel (16) also discovered that maternal FA supplementation can reduce the risk of CAKUT. However, the Canfield (7) study also suggested that FA use is associated with obstructive urinary tract dysplasia, resulting in an increased prevalence; Godwin et al. (8) reported an increase in ureteral obstruction with renal pelvis with FA use (OR =1.45, 95% CI, 1.24–1.70). All of the above findings suggest that whether maternal FA supplementation during pregnancy affects congenital renal and urinary tract malformations in offspring is uncertain. Our final results confirmed that maternal FA supplementation might increase the CAKUT incidence in offspring (OR =1.14; 95% CI, 1.02–1.27; P=0.02). Further subgroup analysis of the CAKUT phenotype in offspring included three groups (hydronephrosis, renal a/dysgenesis, and cystic kidney), and confirmed that maternal FA supplementation/maternal FA enhancement was related to an increased hazard of cystic kidney in offspring. Although there was only one randomised controlled trial (RCT) and one cohort study included in our research, they were nevertheless suggestive of the results. Caution should be exercised in recommending maternal FA supplementation and in selecting the appropriate dose in patients with a family history of CAKUT.
Currently, in Europe, including Italy, the public health recommendation is that healthy women of childbearing age planning to become pregnant should take an FA supplement containing 0.4 mg per day for at least 4 weeks before conception until 8 to 12 weeks of pregnancy for NTD prevention (18). In addition, the recommendation for preventing NTDs in mothers with epilepsy or diabetes is to take FA 4 to 5 mg per day, which means a higher dose of FA supplementation. European countries have not established mandatory FA-fortified food, and enhanced sources of folate can only be obtained through FA supplementation, voluntary fortification, and folate-rich vegetables and fruits. Recent studies have not yet determined optimal levels of serum folate for NTD prevention. However, some retrospective studies (19) have shown a dose-dependent approach to NTD prevention, showing that FA 0.4 mg per day reduces the risk of NTDs by approximately 36%, while FA 5 mg per day reduces the risk by approximately 85%. However, such FA supplementation doses have not been clarified regarding other birth defects, such as CAKUT and congenital heart disease. Bortolus (20) designed a multicenter, double-blind RCT comparing the effect of periconceptional FA supplementation at a higher dose (4 mg per day) versus a conventional dose (0.4 mg per day) on reducing offspring birth defects and adverse reproductive outcomes and found no significant difference in the occurrence of offspring birth defects between the two groups (RR =0.80; 95% CI, 0.31 to 2.03); however, 4 mg/day of FA supplementation reduced the incidence of other adverse pregnancy outcomes (RR =0.51; 95% CI, 0.40 to 0.68). To explore the relationship between different FA doses and the occurrence of renal malformations in offspring, this study also conducted a subgroup analysis of the literature with a clear description of the dose of FA and divided the amount of folic acid supplementation into 6 groups: ≤0.2 mg per day, >0.2 mg per day, ≤0.4 mg per day, >0.4 mg per day, ≤0.6 mg per day, and >0.6 mg per day. Unfortunately, no dose-dependent effect was found among the different folic acid dose supplementation groups. The relationship between the FA dose and the occurrence of CAKUT remains unclear and needs further research.
Our study has several advantages. First, this was the first systematic analysis to clarify the relationship between maternal FA supplementation and renal urinary system developmental malformations in offspring. Our study included high-quality articles with large study populations. We graded the evidence for outcomes using the GRADE system. In our studies, benign pregnancy outcomes for renal dysplasia included hydronephrosis, congenital renal dysplasia, and cystic kidney; folic acid doses were also classified. Furthermore, detailed subgroup analyses were conducted to elucidate the correlation of maternal FA supplementation with offspring renal urinary system developmental malformations.
Of course, our study had some limitations. First, few articles describe the effect of maternal FA supplementation on renal urinary system developmental malformations, and multiple birth defects, including renal dysplastic outcomes, are often reported in one article. At the same time, when we analyzed the effects of maternal FA supplementation on specific renal developmental malformations, the data were limited, and heterogeneity in the outcomes was somewhat difficult to control. We pooled effect sizes using a random-effects model and performed subgroup and sensitivity analyses for primary outcomes. Second, due to the limited literature for final inclusion, this article could not clearly distinguish between FA supplementation before and throughout pregnancy or in early, middle and late pregnancy, making it a possible critical window for further exploration of the effect of FA supplementation on renal dysplasia. Third, the FA supplementation also includes different national and regional FA-fortification policies, including FA supplements, voluntary fortification, and folate-rich vegetables and fruits, which makes it difficult to ensure voluntary and normative fortification of national and regional populations, which may lead to some misclassification of the exposure group. Fourth, there are many genes and potential drug or medication interactions possibly related to CAKUT incidence, which were not considered in this study. Finally, one cohort study and one RCT were included here, which is an important reason for the relatively low level of GRADE evidence. More prospective or experimental studies are needed to provide a higher level of evidence to support the conclusions.
Conclusions
In conclusion, despite the limitations, maternal FA supplementation may be associated with offspring CAKUT, especially cystic kidney. Prescription of FA supplementation for pregnant women should be carefully censored, especially for pregnant mothers with a family history of kidney disease.
Acknowledgments
Funding: This work was supported by the Shanghai “Science and Technology Innovation Action Plan” (Sailing Special Project) (No. 22YF1403600).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-23-11/rc
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-23-11/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-23-11/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vivante A, Hildebrandt F. Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol 2016;12:133-46. [Crossref] [PubMed]
- Jain S, Chen F. Developmental pathology of congenital kidney and urinary tract anomalies. Clin Kidney J 2019;12:382-99. [Crossref] [PubMed]
- Yu M, Tan L, Chen J, et al. Intrauterine low-protein diet disturbs metanephric gene expression and induces urinary tract developmental abnormalities in mice. Biochem Biophys Res Commun 2019;513:732-9. [Crossref] [PubMed]
- Wilkinson LJ, Neal CS, Singh RR, et al. Renal developmental defects resulting from in utero hypoxia are associated with suppression of ureteric β-catenin signaling. Kidney Int 2015;87:975-83. [Crossref] [PubMed]
- Hokke SN, Armitage JA, Puelles VG, et al. Altered ureteric branching morphogenesis and nephron endowment in offspring of diabetic and insulin-treated pregnancy. PLoS One 2013;8:e58243. [Crossref] [PubMed]
- Groen In 't Woud S, Renkema KY, Schreuder MF, et al. Maternal risk factors involved in specific congenital anomalies of the kidney and urinary tract: A case-control study. Birth Defects Res A Clin Mol Teratol 2016;106:596-603. [Crossref] [PubMed]
- Canfield MA, Collins JS, Botto LD, et al. Changes in the birth prevalence of selected birth defects after grain fortification with folic acid in the United States: findings from a multi-state population-based study. Birth Defects Res A Clin Mol Teratol 2005;73:679-89. [Crossref] [PubMed]
- Godwin KA, Sibbald B, Bedard T, et al. Changes in frequencies of select congenital anomalies since the onset of folic acid fortification in a Canadian birth defect registry. Can J Public Health 2008;99:271-5. [Crossref] [PubMed]
- Bower C, Miller M, Payne J, et al. Folate intake and the primary prevention of non-neural birth defects. Aust N Z J Public Health 2006;30:258-61. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009;3:e123-30. [PubMed]
- Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. New York: University of York, 2008.
- American Dietetic Association. ADA Quality Criteria Checklist: Primary Research. American Dietetic Association: Chicago, IL, USA, 2008.
- Gildestad T, Bjørge T, Haaland ØA, et al. Maternal use of folic acid and multivitamin supplements and infant risk of birth defects in Norway, 1999-2013. Br J Nutr 2020;124:316-29. [Crossref] [PubMed]
- Czeizel AE, Dobó M, Vargha P. Hungarian cohort-controlled trial of periconceptional multivitamin supplementation shows a reduction in certain congenital abnormalities. Birth Defects Res A Clin Mol Teratol 2004;70:853-61. [Crossref] [PubMed]
- de Walle HE, Reefhuis J, Cornel MC. Folic acid prevents more than neural tube defects: a registry-based study in the northern Netherlands. Eur J Epidemiol 2003;18:279-80. [Crossref] [PubMed]
- Czeizel AE. Periconceptional folic acid containing multivitamin supplementation. Eur J Obstet Gynecol Reprod Biol 1998;78:151-61. [Crossref] [PubMed]
- Botto LD, Lisi A, Bower C, et al. Trends of selected malformations in relation to folic acid recommendations and fortification: an international assessment. Birth Defects Res A Clin Mol Teratol 2006;76:693-705. [Crossref] [PubMed]
- Cawley S, Mullaney L, McKeating A, et al. A review of European guidelines on periconceptional folic acid supplementation. Eur J Clin Nutr 2016;70:143-54. [Crossref] [PubMed]
- Wald NJ, Law MR, Morris JK, et al. Quantifying the effect of folic acid. Lancet 2001;358:2069-73. [Crossref] [PubMed]
- Bortolus R, Filippini F, Cipriani S, et al. Efficacy of 4.0 mg versus 0.4 mg Folic Acid Supplementation on the Reproductive Outcomes: A Randomized Controlled Trial. Nutrients 2021;13:4422. [Crossref] [PubMed]
Cite this article as: Yu M, Shen Q. Effects of maternal folic acid supplementation on renal urinary system development in human offspring—a meta-analysis and systemic review. Pediatr Med 2023;6:12.