
A narrative review: cardiovascular aspects of Turner syndrome in the pediatric population
Introduction
Turner syndrome (TS) is characterized by a set of phenotypic characteristics in female patients that results from variable penetrance of either complete or partial absence of an X-chromosome. The published incidence within the general population is variable, but is generally agree to be between 1 in 2,500–3,000 live births (1). About half of TS patients have complete absence of an X chromosome, whereas the other half have either duplication of the long arm of one X chromosome or somatic mosaicism. Mosaicism is presence of two or more cell populations with distinct genotypes within one person, and this occurs due to nondisjunction after meiosis during the female embryo development. This results in a portion of the affected individual’s somatic cells having the normal XX while others are X0. The timing of this nondisjunction affects the percentage and distribution of the aneuploid cells within the body of the individual with TS. Mosaic TS patients tend to have less frequent and/or milder forms of congenital heart disease (CHD) than their full monosomy counterparts (2).
The overall prevalence of congenital heart disease in TS patients is 20% to 50%; however, the prevalence and severity of cardiac abnormalities tends to be less in patients with mosaicism (3). Congenital heart disease accounts for 8% and acquired heart disease 41% of the respective mortality in this population, and together, cardiovascular disease is the largest risk factor for death in these patients. About half of the excess risk in TS is due to CHD with an estimated 3-fold increase in early mortality compared to the general population (4). Due to this increased risk, the latest American Heart Association (AHA) guidelines recommend prenatal confirmation by fetal echocardiogram in all cases in which TS is suspected on routine fetal ultrasound. In cases of confirmed CHD, the mother should receive counselling from a pediatric cardiologist to review the potential consequences of the specific defects and to discuss the recommended mode and location of delivery. After delivery, the pediatric cardiology team should be involved in the care of any infant newly diagnosed with TS in order to start a comprehensive evaluation and management plan as soon as possible (5).
In this narrative review, we provide an overview of the care of TS patients from a cardiac perspective. This includes the definitions, evaluation of, and management of the most common cardiac abnormalities that occur in TS patients. The objective of this review is to summarize the current knowledge about the cardiovascular aspects of TS. We present this article in accordance with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-21-101/rc).
Methods and results
A literature review was performed on PubMed using search terms involving the cardiovascular aspects of pediatric patients with TS. A total of 246 English-language articles were found on an initial search. This initial search was narrowed by restricting the search to articles published within the past 20 years, since 2003 (Table 1). From this search, the most relevant articles for use in this review were selected at the discretion of the authors. The search was then narrowed to include only those English language primary sources published within the last 5 years since the 2018 American Heart Association Scientific Statement published. The selected articles we chosen to provide an overview of several topics, including the prevalence of congenital heart and acquired heart disease, electrophysiologic abnormalities, and surgical management and outcomes in TS patients. Additional papers were found via references from articles related to the original search as well, and these were included as deemed appropriate by the authors.
Table 1
| Items | Specification |
|---|---|
| Date of search | Multiple dates |
| Databases and other sources searched | PubMed, MeSH |
| Search terms used | Turner syndrome and cardiovascular; turner syndrome and blood pressure; Turner syndrome and bicuspid aortic valve; Turner syndrome and coarctation of aorta; Turner syndrome and aortic dilation; Turner syndrome and electrocardiogram; turner syndrome and surgical outcomes; Turner syndrome and single ventricle; Turner syndrome and transplantation |
| Timeframe | 2003–2023 |
| Inclusion criteria | Clinical trial; meta-analysis; randomized controlled trial; review; systematic review; language: English |
| Selection process | All authors conducted the searchers at various times; all authors agreed upon the final inclusion of selected articles |
Discussion
Definitions and prevalence of congenital heart disease in TS
There is a significantly increased prevalence of congenital CHD in TS patients, estimated to be anywhere 23% to 70%. This wide variability in the reported prevalence likely reflects a wide array of imaging modalities utilized in the various studies, different interpretation techniques utilized (e.g., MRI, echocardiogram), the spectrum of CHD included in the studies, and differing percentages of the various TS karyotypes represented within the studies (6). For instance, it has been recognized that MRI is better at identifying bicuspid aortic valve than echocardiogram, and so studies utilizing this modality are likely to report a higher prevalence of this particular congenital heart defect (7). Patients with mosaicism are also now known to have lower prevalence of CHD than 45,X patients, and so different percentages of mosaic individuals included in any particular study will affect the percentage of CHD reported. The standardized mortality ratios (SMR) for cardiovascular morbidity and mortality in TS patients is overall 3-fold the general population, in large part due to this higher prevalence of CHD. Within the TS population, SMRs of 20.7 for congenital heart disease, 23.6 for aortic dilation and dissection, 2.8 for ischemic heart disease, and 3.9 for cerebrovascular disease are reported (6). In one study, the investigators used MRI on 99 TS patients in order to determine the prevalence of intrathoracic artery abnormalities, and they found the relative risk for any congenital heart abnormality to be 7.7, with the 2 most common findings being bicuspid aortic valve (BAV) in 27% and aortic coarctation in 13% (8). This is significantly higher than the general population in which BAV occurs in 1–2% and coarctation of the aorta (CoA) occurs in 0.04% of the general population (9). BAV occurs at a higher rate in TS patients than any other syndrome. The frequency of BAV and other left-sided obstructive heart lesions is less in mosaic TS patients and also appears to be different depending on the degree of mosaicism present (2).
The severity of left-sided obstruction is highly variable, with BAV without any valvar stenosis at the mildest end and hypoplastic left heart syndrome (HLHS) at the furthest end of the spectrum of severity. HLHS is characterized by underdevelopment of the left ventricle to the point of being unable to provide adequate systemic cardiac output along with varying degrees of mitral and aortic valve stenosis or complete atresia. A prevalence of 1% to 7% of patients with HLHS also have TS, with the actual prevalence during fetal life likely much higher due to the high rate of fetal demise in patients with this combination of disorders (10).
Bicuspid aortic valve is defined as the presence of 2 aortic valve commissures, indicating the presence of only 2 aortic cusps rather than the typical 3. It is important to note that TS patients are at risk of having congenital BAV in which 2 cusps are fused during the development of the valve in utero rather than acquired BAV that can occur due to fibrosis and calcification later in life. BAV can result in varying degrees of stenosis or regurgitation that is typically progressive because of the higher tendency for calcification and thickening of the valve cusps over time. When BAV is present in the context of TS, it is characterized as a complex valvulo-aortopathy due to the higher likelihood of accelerated aortic valve dysfunction (9). Due to the limitation of echo resolution, BAV can be quite difficult to diagnose early in life and is now occasionally captured by MRI later in life.
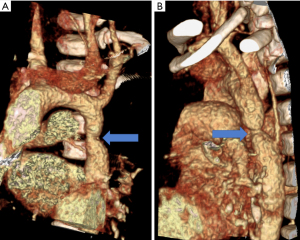
Neck-webbing has been found to be highly predictive of both BAV and CoA with a 45% coincidence, whereas only 23% of patients who did not have neck-webbing have these cardiac defects (7,11). Lymphedema and the subsequent development of neck-webbing are hallmark features of TS and are associated with the development of CHD in utero. Patients with lymphedema noted on fetal ultrasound have a higher rate of severe CHD than those without. In theory, the increased lymphatic pressure from jugular lymphatic sac obstruction may cause distension of the thoracic duct and compression of the ascending aorta. This decreases the amount of intracardiac blood flow in the developing fetal heart and may result in left-sided cardiac obstructive lesions (12-14). CoA is a narrowing or stenosis at the level of the isthmus of the aorta, which is located immediately distal to the takeoff of the left subclavian artery, resulting in obstruction to blood flow to the body distal to takeoff of the head and neck arteries (Figure 1). Studies have found similar prevalences of BAV and CoA in TS patients of 14–30% and 7–25% respectively, and these defects are often found together on initial screening. A study has also found, however, that CoA can occur later in life in TS patients and may only be found due to clinical suspicion or on serial MRI’s over several years. This is contradictory to the previously accepted theory that CoA occurs solely during embryologic development (5,15,16).
Other cardiovascular defects that are associated with TS include partial anomalous pulmonary venous connection (PAPVC) with a prevalence of 1–15% (14,15,17), atrial and ventricular septal defects seen in 1–8% (15), left superior vena cava (LSVC) seen in 1–13% (15), and finally mitral valve abnormalities found in less than 5% of patients (16).
Acquired cardiac diseases
Aortic dilation is a known feature of TS and occurs at a much higher rate than the general population. Aortic dissections occur rarely in patient with TS at a rate of 36 per 100,000 person-years; however, this is much greater than the 6 per 100,000 person-years within the general population. It is estimated that at least 1.4 per 100 TS patients will have an aortic dissection in their lifetime. The median age of dissection is 35 years in these women, but with a very wide range from 4 to 65 years (18). Aortic dissection in TS patients mostly commonly originates in the ascending aorta (type A dissection), after dilation of this portion of the aorta, and this is more prevalent in TS patients with the 45X/46X,Y karyotype (16,19). The incidence of aortic dissection peaks between the ages of 29–35 years. Even children and adolescents with TS have an increased risk with an incidence of 14 per 100,000 TS patients per year between ages 0–19 years (5,6,19). Hypertension is the most consistent risk factor for dissection in TS patients, but bicuspid or unicuspid aortic valve and/or CoA have also been found to be highly contributory (5,15,19-21). In a single center study of 20 TS patients with type A aortic dissection, 18 had bicuspid aortic valve and a third had hypertension. The average age of dissection in this particular study was 31.5 years (18). While hypertension has clearly been identified as a risk factor for dissection in TS patients, this does not completely explain the increased prevalence of dissection over the general population in which hypertension is also common. Medial cystic necrosis of the aorta has been noted on autopsies of TS patients and this has been long felt to be the most likely cause for aortic dilation and ultimate dissection in TS patients.
As previously stated, patients with TS are at increased risk of developing acquired cardiovascular disease as well, particularly from the secondary effects of hypertension (5,19). Hypertension occurs at a much higher rate in patients with TS than the general population, with an estimated 20–40% prevalence during childhood that increases to 60% by adulthood. Hypertension in these patients can occur secondary to CoA, although in most cases, a definitive cause is never identified. In one study, the prevalence of hypertension, defined as a systolic blood pressure >95th percentile, was 51.7% in those with a history of CoA repair versus 32.4% who did not have CoA (22). It has been hypothesized that CoA may result in pre- and post-natal renal hypoperfusion, resulting in pathologic changes, such as dysregulation of neuroendocrine hormones, that impact long-term blood pressure control. Additionally, TS patients are known to have a particular form of vasculopathy characterized by thickening of the intimal and medial layers of the aortic wall, and this has been thought to be a potential cause for hypertension in these patients. Pulse wave velocity (PWV) calculated from the carotid to the femoral artery along with augmentation index (AI) are both standard methods for determining arterial stiffness. Despite the known increase in intima-medial wall thickness (IMT) in patients with TS, a comparative study of 93 TS patients failed to find a difference in PWV or AI between matched controls and the TS patients (23). Another study also found little difference in aortic distensibility between TS patients and controls, except for those with a history of CoA in which central diastolic pressure was also significantly higher (24). These studies leave major questions regarding the origin of hypertension in TS patients without CoA. Additionally, the lack of difference in PWV and AI in TS patients compared to controls does not illuminate any potential etiology for their aortic dilation.
Considering the deleterious effects of hypertension on aortic dilatation, current TS guidelines recommend the maintenance of normal blood pressure through medical therapy (18). Autopsies have revealed cystic intima-medial degeneration within the aortic wall in TS patients that is similar to that seen in other patients with aortopathies or connective tissue disorders, such as Marfan Syndrome. It is thought that these aortic wall abnormalities are likely underlying the aortic dilation seen in TS. Studies on Marfan syndrome patients have found that beta blockers and angiotensin receptor blockers are similarly beneficial at reducing not only blood pressure, but also the rate of aortic dilation (25). Lin et al. conducted a study to screen TS patients for a wide array of CHD, and it was found that 80–90% of TS patients with aortic root dilation had underlying hypertension or congenital heart disease, of which BAV was most common. These are both risk factors for the development of aortic root dilation (20). Medical treatments that have been found to reduce the rate of aortic dilation in Marfan patients have begun to be utilized in the treatment of hypertension in TS patients in order to reduce the risk of dissection.
Surveillance and defining risk for aortic dissection/rupture
Patients with TS are at increased risk for associated CHD and the development of acquired heart disease, both of which increase their long-term risk for early morbidity and mortality. If there is suspicion for TS on routine fetal ultrasound, a fetal echocardiogram is recommended to screen for CHD. If CHD is found at this screening, there should be routine follow-up provided by a Pediatric Cardiologist during the pregnancy and a determination of a delivery plan must be made depending on the severity of the defect. If the diagnosis is suspected or confirmed postnatally, the patient should undergo a complete and thorough physical examination, including a detailed cardiac examination, palpation of peripheral pulses, and four extremity blood pressures, an ECG, and a complete echocardiogram (5,17,21). When an otherwise asymptomatic female infant or child is found to have BAV, CoA, or another left-sided obstructive cardiac defect, then genetic testing to evaluate for TS is recommended, in addition to the previously mentioned work-up (5,26). Patients with TS then require more robust screening throughout life due to their increased risk of early onset CHD.
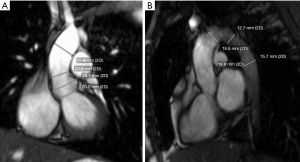
Aortic dilation has historically been difficult to quantify in TS patients. Even the definition of dilation in these patients has been a source of much debate because of different modalities used, methods of measurement within the same modality, and challenges in determining the ideal method to index aortic dimensions to body size. There has been robust debate over the best methodology for measuring aortic dimensions by echocardiogram, particularly due to differences between the pediatric and adult conventions of interpretation. Therefore, there was a push to use standardized methodology across all patients, and most data now for TS patients is based upon studies in which an inner-edge to inner-edge method for both echocardiogram and MRI is used (27-29) (Figure 2). Echocardiogram has several advantages over MRI, including wider availability of both machines and qualified readers, no need for IV contrast, faster scan time, and lower cost. Nevertheless, MRI provides greater spacial resolution and thus, accuracy in the measurement of aortic dimensions, and therefore, this modality is becoming an dispensable part of the surveillance in TS patients. MRI also provides more reproducible measurements, particularly in cases in which the aortic root is asymmetric, as is the case in many TS patients with aortopathy (30).
TS patients are shorter and have lower body surface area (BSA) for their age than the general population (5,26,29). They also tend to have a higher risk of aortic dissection and rupture at lower absolute ascending aorta diameters than non-syndromic patients with aortic dilation, and so determining appropriate cut-offs for medical and surgical intervention has been difficult. Unlike in the general adult population, absolute aortic measurement cut-offs have not been found to be useful in TS patients. In order to address this issue, an index value called the aortic size index (ASI) was developed. This value is obtained by dividing the ascending aortic diameter by the BSA. This was previously the preferred method of quantifying aortic dilation in TS patients over the age of 15 when body size remains fairly constant. Due to the higher aortic diameter to BSA ratio in patients younger than 15, there remains debate as to how best to quantify their aortic dilation. These adolescent patients tend to have a higher ASI that slowly declines with somatic growth. TS girls under the age of 15 commonly have an ASI of >2.5 cm/m2, which is considered high risk in adult patients (31). A study on aortic dimensions by MRI in healthy children demonstrated a linear relationship between absolute diameters and the square root of BSA; however, this has not become standard practice in TS patients (32). ASI also suffers from issues related to over- and under-correction in adult TS patients who are at the extremes of weight, with lower scores in obese patients and higher in slim. The problems with ASI lead Quezada et al. to establish TS-specific reference curves for aortic dimensions based upon echocardiographic imaging data on 481 otherwise healthy TS girls and women (29). The Z-scores based upon this data have now been included in the most recent AHA guidelines (5).
Aortic dissection is one of the most feared outcomes in TS patients and involves tearing of the intimal layer, resulting in a split within the aortic wall that can ultimately lead to rupture of the outer later and extravasation. Blood flow into the false lumen created by this separation of the aortic layers can also lead to compression of the true lumen of the artery and obstruction of blood flow to the body or coronary compression, resulting in myocardial ischemia. The second most common cause of death following aortic dissection is acute aortic valve regurgitation, which affects 40–75% of patients (33). In a cohort of 20 adult TS patients with a history of aortic dissection, 15 had aortic dimensions available by echocardiogram or MRI that showed a mean ASI of 2.7±0.6 cm/m2 (18). One of the largest and most recent studies of aortic dimensions using MRI and involving 268 TS patients found that aortic dilation (defined as ASI >2 cm/m2) was present in one-fifth of patients, but when using a TS-specific Z-score, this increased to 22.8%. Over nearly 7 years of follow-up, the incidence of aortic dissection was quite low at 2% overall or 0.3%/year (29). Based upon data such as this, the current AHA guidelines attempt to risk stratify TS patients in terms of both their other cardiac comorbidities as well as their degree of aortic dilation. For TS patients greater than 15 years old with no known history of comorbidities, including CoA, BAV, or hypertension, there are 3 categories of risk from mild to severe based upon ASI. An ASI below 2 cm/m2 is considered low risk, and the recommendation for these patients is periodic echocardiogram or MRI surveillance every 5 years or even longer at the discretion of the Cardiologist. Above an ASI of 2.3 cm/m2, patients are considered high risk and should undergo surveillance annually. An intermediate ASI between these cut-offs is considered moderate risk with recommended surveillance between every 3 to 5 years. Patients with known comorbidities as previously listed, fall into either moderate or severe risk categories with a cut-off in ASI of 2.3 cm/m2. Below this threshold, patients are considered moderate risk and should have surveillance about as often as those of moderate risk without comorbidities. High-risk patients with comorbidities should have surveillance at least annually. This method of risk stratification based upon both indexed aortic size and co-morbidities seems to be validated by a recent study of surgical outcomes in which all 5 patients who had an aortic dissection also had at least 1 of the 2 risk factors of hypertension or congenital heart disease, of which CoA and BAV were the most common. Dissection occurred primarily in the fifth decade in this cohort, with no occurrences after 2004, which suggests that greater recognition of risk based upon the current guidelines may have lead to earlier intervention (34). Pediatric patients’ risk is based upon the same comorbidities as adults, but instead of ASI, TS-specific z-score should be used to quantify their aortic dilation for the reasons listed above (5).
TS patients without any cardiovascular symptoms or disease should be screened yearly beginning in early childhood for the development of hypertension, and it should be treated based upon current hypertension guidelines. These specify that patients should have a blood pressure <130/80, with systolic blood pressure >120 mmHg as borderline (5,35). Ambulatory blood pressure monitoring (ABPM), in which a mobile blood pressure cuff is worn for 24 h or longer, should be considered in all TS patients with left ventricular hypertrophy or elevated left ventricular mass noted on echocardiogram, even if they have a normal blood pressure reading in the office (5,36). TS patients have been shown to have large fluctuations in blood pressure throughout the day, and therefore, hypertension may go undiagnosed based upon measurements done at a single point in time. Diastolic blood pressure dips that are only detectable by ABPM have also been associated with increased carotid intima-media thickness, which in itself is a risk for insulin resistance and acquired cardiovascular disease (37).
It is of utmost importance that TS patients be followed by a Cardiologist throughout life due to their increased risk. Since the mean age of diagnosis of TS is about 9 years of age, typically these patients are initially treated by a pediatric team; however, one study found that only 32% of patients are transitioned to adult Cardiology care. This resulted in appropriate cardiac imaging surveillance in only 36% of patients. It was also demonstrated that those patients followed by a primary care provider or other subspecialists without Cardiology involvement were at significantly increased risk of aortic dissection. It has been postulated that direct Cardiology involvement was beneficial due to more aggressive and early action on echocardiographic findings (38).
Electrophysiologic findings in TS patients
In addition to CHD and acquired heart disease, about half of TS patients have been estimated to have “abnormalities” on ECG. Prolongation of the QT interval is likely the best studied of these issues, however, accelerated AV conduction, right axis deviation (typically suggestive of left to right shunt), left posterior fascicular block, and other T-wave abnormalities have also been described as being more prevalent in TS patients (39). One of the first systematic studies to describe these abnormalities in 2008 reported on 100 TS patients versus 100 matched controls, and the results were significant for 21% of the TS patients having a prolonged QTc as defined as >440 ms compared to no controls with QTc prolongation (40). A separate study also found the same prevalence of 21% QTc prolongation among 86 TS patients, however, this time it was defined as a QTc >450 ms (41). A study on the QT dispersion, defined as the difference between the longest and shortest QTc on an ECG, and heart rate variation found that TS patients may have autonomic dysfunction with a sympathetic dominance as a potential cause of their ECG abnormalities. This may also explain the elevation in heart rate that is a common feature of TS along with a quickened AV conduction rate as manifested by a shortened PR interval.
Autonomic dysfunction with a sympathetic dominance has previously been associated with an increased risk of ventricular arrhythmias, and so it has been postulated that TS patients may be at increased risk (42). Despite these concerns regarding both sympathetic dominance and QT interval prolongation, no study has ever demonstrated a higher prevalence of documented ventricular arrhythmias in women with TS compared to the general population. This may be due to several factors. For instance, several prior studies have used lower cut-offs for QT compared to the currently recommended 460 ms for women, including those with TS (43). As has been previously noted, QTc changes may be more related to autonomic fluctuations rather than a pathologic channelopathy.
When applied to an electrocardiogram, the ideal QT correction formula should result in a uniformly linear relationship between the QT interval and heart rate within the normal physiologic range of heart rates. This means that the formula should result in the same QTc at any heart rate between 60 and 180 bpm for any given individual. Unfortunately, no such formula has ever existed. The Bazett formula [QT/(RR)1/2] was developed in 1920 and has been used almost universally in the clinical setting to correct the QT interval for heart rate (44). The Bazett formula has more recently been criticized as potentially “overcorrecting” the QT interval at higher heart rates, resulting in spuriously long QTc intervals, especially when compared to other formulae such as the Framingham, Hodges, and or even individualized formulae based upon 24 h Holter data (45,46). Thus, concern has been raised about using the Bazett formula in TS patients, especially during the pediatric years, due to their tendency to have elevated heart rates. The Hodges formula [QT + 1.75 × (HR – 60)] may be superior to the Bazett because it results in a more level correction across higher heart rates; however, there is little to no use of this formula in clinical practice at this time. In 2 studies in which the Hodges formula was compared to the Bazett using a cutoff of 460 ms, there was in fact no difference in QTc between TS patients and controls (47,48). This would suggest that the QT prolongation that has been thought to be a common feature of TS may in fact be a result of inappropriate correction by the formula we typically use on ECG. According to the AHA guidelines, exercise testing is recommended as a screening tool in patients with TS and QT prolongation >460 ms on baseline ECG. It has also been shown, however, that TS women with a prolonged QTc at baseline have normalization of their QTc during exercise testing, which does not occur in cases of true long QT syndrome (49). Holter monitoring is also a useful tool for determining if QT prolongation is persistent in a patient with QT prolongation on baseline ECG (5).
There is little evidence in the literature to suggest that the use of QT prolonging medication places TS patients at increased risk, except for a single case report involving amiodarone (50). Current guidelines continue to suggest that it is reasonable to avoid such medications in cases of baseline QTc prolongation. If such medications cannot be avoided due to perceived benefit exceeding risk, then to obtain an ECG within 1 to 2 weeks after initiation of the medication (5).
Interventional catheterization and surgical management of TS patients and outcomes
As previously discussed, dilation of the ascending aorta with subsequent aneurysm can lead to dissection and rupture in TS patients at a significantly higher rate and at lower aortic size than the general population. Therefore, the purpose of surveillance is to determine if surgical intervention is indicated in order to prevent this nearly universally fatal complication. Although dissection is much more common in TS patients than the general population, it is still rare, and therefore, the consensus on thresholds of indexed aortic size is based upon only a few retrospective studies and case reports largely done by the same group (5,18,26,51). As previously mentioned, adult TS patients who have an ASI >2.3 cm/m2 require frequent surveillance because a slight increase to >2.5 cm/m2 over time, then becomes an indication for prophylactic aortic replacement. If the patient is at the extremes of weight and their BSA is significantly different than the average of 1.6 m2 for TS patients, then a TS-specific z-score of >4 can instead be used. This also applies to pediatric patients <15 years old, who will often have an ASI >2.5 cm/m2 (5,52). Rapid dilation, defined as a rate of dilation >0.5 cm/year is also considered a potential indication for surgery, and more aggressive medical management.
CoA is present in approximately 17% of TS patients and thus, is a common indication for intervention in this population. Correction of CoA can be pursued with cardiac catheterization or surgery. Intervention by catheterization can involve either balloon angioplasty alone or stent placement across the aortic narrowing. It has been theorized that the innate fragility of the aortic wall in TS patients may result in the higher risk associated with aortic stenting. These patients have a higher rate of dissection and rupture than the general population undergoing the same catheter-based procedures. The largest single study to date on TS patients with CoA undergoing stent placement involved 19 patients from 10 centers. While no patients had procedural complications, 5 had adverse events on follow-up, with 3 occurring within 30 days of the procedure. Two patients out of this small cohort had proven dissection and one, who had had a prior surgical intervention on their CoA, died from dissection shortly after stent placement. Overall, the procedure-related risk was much higher than the 1% found in the non-syndromic population (53). In 2010, a review of the literature reported on outcomes from 46 surgical resections, 49 balloon angioplasties, and 15 stent placements as treatment for CoA. This study demonstrated balloon angioplasty to be the safest intervention, with a morbidity and mortality of 2% and 0% compared to surgery that resulted in 30% morbidity and 11% mortality. In this same study, in which 10 patients underwent CoA stenting, aneurysms around the stent occurred in 2 (20%) of the patients. This indicates that while stenting is still an option in these patients, it still comes with significant risk (54). A case report detailing the death of a 19-year-old TS patient from aortic dissection following serial stent dilation also stands as a warning of the danger involved with intervention in these patients (55). While the available data may suggest that balloon angioplasty is the safest option in these patients, the rate of recoarctation after this procedure is significant and the risk becomes cumulative over several attempts at angioplasty, making it a less desirable intervention.
Surgical repair of CoA with an end-to-end anastomosis remains standard of care at most centers, and this is the most frequent surgical procedure performed in TS girls and women (14,56). An analysis of the Society of Thoracic Surgeons (STS) Database by Chew et al. reported on 274 cases of CoA repair accounted for 35% of the overall surgeries that the TS patients. These patients had a longer post-operative length of stay in the hospital compared to their peers without TS (56,57), likely secondary to the significantly higher rate of post-operative complications. For instance, significantly more TS patients were found to develop chylous effusions at 8.8% compared to the 2.8% incidence in the control group. Pleural effusions requiring drainage also occurred at a rate of 3.6% compared to 1% in the control group of patients undergoing aortic arch repair. Despite TS patients having increased risks of post-operative morbidity, it has not been demonstrated that there is an increased risk of operative mortality compared to patients without TS in the cases of surgical CoA repair and aortic arch repair (14,56,57). A recent review of 51 TS patients who underwent cardiac surgery at the Mayo Clinic found that the adult patients had elevated post-operative risk due to their higher medical complexity and cardiovascular risk factors; however, the pediatric patients did not seem to have an elevated risk of mortality or need for reoperation compared to the general population (34). Perhaps this difference in post-operative complications in this more modern cohort is due to the increased recognition of the complexities these patients pose.
In cases of more severe left heart obstruction such as HLHS, more complex surgical intervention is necessary. Often the only option for these patients is to proceed down a single ventricle palliative pathway that involves 3 surgeries early in life, including the Norwood procedure (stage I), Glenn procedure (stage II), and finally Fontan completion (stage III). An in-depth description of these complex surgeries is beyond the purview of this review; however, the goal of this staged palliative pathway is to reroute all systemic venous blood flow directly to the pulmonary arteries, without a sub-pulmonary ventricle. This allows the morphologic right ventricle to be the systemic pumping chamber alone, since in HLHS the morphologic left ventricle is too small to provide cardiac output to the body. HLHS patients undergoing staged palliation are most tenuous between stage I and stage II, which is called the interstage period. TS patients with HLHS have an increased risk of mortality after undergoing single ventricle palliation when compared to patients without TS. Cramer et al. reported their single center data at the Children’s Hospital of Wisconsin in which 4 patients underwent a Norwood procedure. In this series, 1 patient died before making it to stage II palliation and of the 3 patients remaining, only 1 patient ultimately survived as a Fontan patient. This patient was in palliative care at the time of publication (57). The Texas Birth Defect Registry was also queried by Lara et al., and this study reported the outcomes of 11 patients with HLHS and TS. In this series, only 5 patients survived to stage I palliation, and then 3 died during the interstage period after Norwood procedure. Two underwent stage II palliation and survived for one year following the procedure, but then 1 then died, leaving only 1 survivor at the time of the study (10). The previously-mentioned STS database study reported on 59 TS patients who underwent the Norwood procedure and there was no difference in operative mortality, cardiopulmonary bypass time, major post-operative complications, or post-operative length of stay between TS patients and other infants with HLHS. Nevertheless, there was a noticeable drop in the number of TS patients reported to have undergone Glenn operation to 33 and there was a further decline to 27 who underwent Fontan completion. This contrasts with the general HLHS patient population, in which there were in fact more patients who underwent Glenn and Fontan then Norwood since at times there is no need for Norwood as the initial surgery. Due to the limitation of such a review, it was impossible to know for sure the reason for this decline in the number of TS patients over the course of the 3 stages, but it was postulated that it could be due to interstage mortality (56). A separate study on the outcomes of surgical intervention in patients with HLHS demonstrated that genetic syndromes, of which TS was the most common in the cohort, confers a highly unfavorable risk of adverse events and death following the Norwood operation with mortality reported as high as 80% (58). A recent study utilizing the Pediatric Cardiac Care Consortium registry data reported on the outcomes of 179 pediatric-aged TS patients with left-sided obstructive lesions, including CoA, aortic valve stenosis, and HLHS variants, found that the overall 30-year transplant free survival was 90.4% and not different from matched controls. Despite this, the survival of TS patients with HLHS seemed to be much lower than the controls, with only 33% survival versus 51% in the nonsyndromic patients (59). Due to the increased risk TS patients with HLHS pose for staged palliation, the question of primary transplant has previously been raised. A case report on a patient with TS and HLHS described the clinical course that involved the patient undergoing successful heart transplantation following a temporizing hybrid procedure (60). Further research is necessary to more thoroughly evaluate this as a potential option in these patients given the inherently higher risk they have with the more standard staged single ventricle palliation.
Conclusions
Patients with TS are at an increased risk of being born with CHD, typically left-sided obstructive cardiac lesions, including BAV and CoA of the aorta, and are at increased risk for developing acquired heart disease during their lifetime, including hypertension, ascending aortic dilation, and aortic dissection. A concern for TS on prenatal screening should prompt a complete cardiac evaluation prior to birth if possible with continued surveillance throughout life. The frequency at which screening takes place should be based depending upon their risk assessments over time. Pediatric cardiology, and often Cardiothoracic Surgery, need to be involved in the care of any TS patient with congenital or acquired heart defects. Once these patients reach their adult years, they should be transitioned to adult Cardiology for continued surveillance. Electrocardiographic abnormalities are seen more often in these patients as well, however, there is currently no data to suggest that they are at increased risk for fatal arrhythmias. Medical management of these patients from a cardiovascular standpoint mostly centers upon treatment of hypertension, which is more common in this population and can accelerate aortic dilation. Surgical management is often necessary for TS patients born with left-sided obstructive lesions or those who develop a concerning degree of aortic dilation that places them at increased risk for dissection. Despite having increased risk for prolonged hospital stay and minor complications, studies have shown that TS patients have similar long-term outcomes following surgery as their nonsyndromic peers. The exception to this is TS patients with HLHS who seem to fare worse than their nonsyndromic peers in the course of staged surgical palliation. Due to the increased risk that TS confers in cases of HLHS, some would consider primary transplant a better option.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Berrin Ergun-Longmire, Ethel Clemente, and Patricia Vining-Maravolo) for the series “Clinical Pearls in Pediatric Endocrinology and Metabolism” published in Pediatric Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-21-101/rc
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-21-101/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-21-101/coif). The series “Clinical Pearls in Pediatric Endocrinology and Metabolism” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sybert VP, McCauley E. Turner's syndrome. N Engl J Med 2004;351:1227-38. [Crossref] [PubMed]
- Prakash SK. The impact of somatic mosaicism on bicuspid aortic valve and aortic dissection in Turner Syndrome. Am J Med Genet C Semin Med Genet 2019;181:7-12. [Crossref] [PubMed]
- Digilio MC, Calcagni G, Unolt M, et al. Congenital Cardiac Disease in the Setting of Genetic Syndromes. In: Wernovsky G, Anderson RH, Kumar K, et al. editors. Anderson's Pediatric Cardiology. 4th ed. Philadelphia, PA: Elsevier; 2020: 1407-20.
- Schoemaker MJ, Swerdlow AJ, Higgins CD, et al. Mortality in women with turner syndrome in Great Britain: a national cohort study. J Clin Endocrinol Metab 2008;93:4735-42. [Crossref] [PubMed]
- Silberbach M, Roos-Hesselink JW, Andersen NH, et al. Cardiovascular Health in Turner Syndrome: A Scientific Statement From the American Heart Association. Circ Genom Precis Med 2018;11:e000048. [Crossref] [PubMed]
- Mortensen KH, Andersen NH, Gravholt CH. Cardiovascular phenotype in Turner syndrome--integrating cardiology, genetics, and endocrinology. Endocr Rev 2012;33:677-714. [Crossref] [PubMed]
- Sachdev V, Matura LA, Sidenko S, et al. Aortic valve disease in Turner syndrome. J Am Coll Cardiol 2008;51:1904-9. [Crossref] [PubMed]
- Mortensen KH, Hjerrild BE, Andersen NH, et al. Abnormalities of the major intrathoracic arteries in Turner syndrome as revealed by magnetic resonance imaging. Cardiol Young 2010;20:191-200. [Crossref] [PubMed]
- Michelena HI, Della Corte A, Evangelista A, et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. J Thorac Cardiovasc Surg 2021;162:e383-414. [Crossref] [PubMed]
- Lara DA, Ethen MK, Canfield MA, et al. A population-based analysis of mortality in patients with Turner syndrome and hypoplastic left heart syndrome using the Texas Birth Defects Registry. Congenit Heart Dis 2017;12:105-12. [Crossref] [PubMed]
- Loscalzo ML, Van PL, Ho VB, et al. Association between fetal lymphedema and congenital cardiovascular defects in Turner syndrome. Pediatrics 2005;115:732-5. [Crossref] [PubMed]
- Clark EB. Neck web and congenital heart defects: a pathogenic association in 45 X-O Turner syndrome? Teratology 1984;29:355-61. [Crossref] [PubMed]
- Berdahl LD, Wenstrom KD, Hanson JW. Web neck anomaly and its association with congenital heart disease. Am J Med Genet 1995;56:304-7. [Crossref] [PubMed]
- Morales-Demori R. Congenital heart disease and cardiac procedural outcomes in patients with trisomy 21 and Turner syndrome. Congenit Heart Dis 2017;12:820-7. [Crossref] [PubMed]
- Bondy CA. Congenital cardiovascular disease in Turner syndrome. Congenit Heart Dis 2008;3:2-15. [Crossref] [PubMed]
- Goldmuntz E, Crenshaw ML. Genetic Aspects of Congenital Heart Defects. Moss and Adam' Heart Disease in Infants, Children, and Adolescents. Ninth Edition ed. Philadelphia, PA: Wolters Kluwer; 2016: 87-115.
- Mazzanti L, Cacciari E. Congenital heart disease in patients with Turner's syndrome. Italian Study Group for Turner Syndrome (ISGTS). J Pediatr 1998;133:688-92. [Crossref] [PubMed]
- Carlson M, Airhart N, Lopez L, et al. Moderate aortic enlargement and bicuspid aortic valve are associated with aortic dissection in Turner syndrome: report of the international turner syndrome aortic dissection registry. Circulation 2012;126:2220-6. [Crossref] [PubMed]
- Gravholt CH, Landin-Wilhelmsen K, Stochholm K, et al. Clinical and epidemiological description of aortic dissection in Turner's syndrome. Cardiol Young 2006;16:430-6. [Crossref] [PubMed]
- Lin AE, Lippe B, Rosenfeld RG. Further delineation of aortic dilation, dissection, and rupture in patients with Turner syndrome. Pediatrics 1998;102:e12. [Crossref] [PubMed]
- Dulac Y, Pienkowski C, Abadir S, et al. Cardiovascular abnormalities in Turner's syndrome: what prevention? Arch Cardiovasc Dis 2008;101:485-90. [Crossref] [PubMed]
- Los E, Quezada E, Chen Z, et al. Pilot Study of Blood Pressure in Girls With Turner Syndrome: An Awareness Gap, Clinical Associations, and New Hypotheses. Hypertension 2016;68:133-6. [Crossref] [PubMed]
- Ostberg JE, Donald AE, Halcox JP, et al. Vasculopathy in Turner syndrome: arterial dilatation and intimal thickening without endothelial dysfunction. J Clin Endocrinol Metab 2005;90:5161-6. [Crossref] [PubMed]
- Wen J, Trolle C, Viuff MH, et al. Impaired aortic distensibility and elevated central blood pressure in Turner Syndrome: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2018;20:80. [Crossref] [PubMed]
- Teixido-Tura G, Forteza A, Rodríguez-Palomares J, et al. Losartan Versus Atenolol for Prevention of Aortic Dilation in Patients With Marfan Syndrome. J Am Coll Cardiol 2018;72:1613-8. [Crossref] [PubMed]
- Mortensen KH, Young L, De Backer J, et al. Cardiovascular imaging in Turner syndrome: state-of-the-art practice across the lifespan. Heart 2018;104:1823-31. [Crossref] [PubMed]
- Bossone E, Yuriditsky E, Desale S, et al. Normal Values and Differences in Ascending Aortic Diameter in a Healthy Population of Adults as Measured by the Pediatric versus Adult American Society of Echocardiography Guidelines. J Am Soc Echocardiogr 2016;29:166-72. [Crossref] [PubMed]
- Kim HK, Gottliebson W, Hor K, et al. Cardiovascular anomalies in Turner syndrome: spectrum, prevalence, and cardiac MRI findings in a pediatric and young adult population. AJR Am J Roentgenol 2011;196:454-60. [Crossref] [PubMed]
- Quezada E, Lapidus J, Shaughnessy R, et al. Aortic dimensions in Turner syndrome. Am J Med Genet A 2015;167A:2527-32. [Crossref] [PubMed]
- Nejatian A, Yu J, Geva T, et al. Aortic Measurements in Patients with Aortopathy are Larger and More Reproducible by Cardiac Magnetic Resonance Compared with Echocardiography. Pediatr Cardiol 2015;36:1761-73. [Crossref] [PubMed]
- Corbitt H, Maslen C, Prakash S, et al. Allometric considerations when assessing aortic aneurysms in Turner syndrome: Implications for activity recommendations and medical decision-making. Am J Med Genet A 2018;176:277-82. [Crossref] [PubMed]
- Kaiser T, Kellenberger CJ, Albisetti M, et al. Normal values for aortic diameters in children and adolescents--assessment in vivo by contrast-enhanced CMR-angiography. J Cardiovasc Magn Reson 2008;10:56. [Crossref] [PubMed]
- Sayed A, Munir M, Bahbah EI. Aortic Dissection: A Review of the Pathophysiology, Management and Prospective Advances. Curr Cardiol Rev 2021;17:e230421186875. [Crossref] [PubMed]
- Fuchs MM, Attenhofer Jost CH, Said SM, et al. Cardiovascular surgery in Turner syndrome - early outcome and long-term follow-up. World J Cardiol 2020;12:97-106. [Crossref] [PubMed]
- Saenger P. Turner's syndrome. N Engl J Med 1996;335:1749-54. [Crossref] [PubMed]
- Fudge EB, Constantacos C, Fudge JC, et al. Improving detection of hypertension in girls with turner syndrome using ambulatory blood pressure monitoring. Horm Res Paediatr 2014;81:25-31. [Crossref] [PubMed]
- Akyürek N, Atabek ME, Eklioglu BS, et al. Ambulatory blood pressure and subclinical cardiovascular disease in children with turner syndrome. Pediatr Cardiol 2014;35:57-62. [Crossref] [PubMed]
- Yetman AT, Bisselou KSM, Sanmann JN, et al. Vascular dissection in women with Turner syndrome. Int J Cardiol 2021;325:127-31. [Crossref] [PubMed]
- Bondy CA, Ceniceros I, Van PL, et al. Prolonged rate-corrected QT interval and other electrocardiogram abnormalities in girls with Turner syndrome. Pediatrics 2006;118:e1220-5. [Crossref] [PubMed]
- Bondy CA, Van PL, Bakalov VK, et al. Prolongation of the cardiac QTc interval in Turner syndrome. Medicine (Baltimore) 2006;85:75-81. [Crossref] [PubMed]
- Dalla Pozza R, Bechtold S, Kääb S, et al. QTc interval prolongation in children with Ulrich-Turner syndrome. Eur J Pediatr 2006;165:831-7. [Crossref] [PubMed]
- Sozen AB, Cefle K, Kudat H, et al. Atrial and ventricular arryhthmogenic potential in Turner Syndrome. Pacing Clin Electrophysiol 2008;31:1140-5. [Crossref] [PubMed]
- Rautaharju PM, Surawicz B, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009;53:982-91. [Crossref] [PubMed]
- Bazett H. An analysis of time-relations of the electrocardiogram. Heart 1920;7:353-70.
- Molnar J, Weiss J, Zhang F, et al. Evaluation of five QT correction formulas using a software-assisted method of continuous QT measurement from 24-hour Holter recordings. Am J Cardiol 1996;78:920-6. [Crossref] [PubMed]
- Robyns T, Willems R, Vandenberk B, et al. Individualized corrected QT interval is superior to QT interval corrected using the Bazett formula in predicting mutation carriage in families with long QT syndrome. Heart Rhythm 2017;14:376-82. [Crossref] [PubMed]
- Noordman ID, Duijnhouwer AL, Coert M, et al. No QTc Prolongation in Girls and Women with Turner Syndrome. J Clin Endocrinol Metab 2020;105:e4148-56. [Crossref] [PubMed]
- Harrahill NJ, Yetman AT, Danford DA, et al. The QT Interval in Patients With the Turner Syndrome. Am J Cardiol 2021;140:118-21. [Crossref] [PubMed]
- Dalla Pozza R, Bechtold S, Urschel S, et al. QTc interval prolongation in children with Turner syndrome: the results of exercise testing and 24-h ECG. Eur J Pediatr 2009;168:59-64. [Crossref] [PubMed]
- Nielsen DG, Nielsen JC, Trolle C, et al. Prolonged QT interval and cardiac arrest after a single dose of amiodarone in a woman with Turner's syndrome. Clin Case Rep 2017;5:154-8. [Crossref] [PubMed]
- Carlson M, Silberbach M. Dissection of the aorta in Turner syndrome: two cases and review of 85 cases in the literature. BMJ Case Rep 2009;2009:bcr0620091998. [Crossref] [PubMed]
- Gravholt CH, Andersen NH, Conway GS, et al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol 2017;177:G1-70. [Crossref] [PubMed]
- van den Hoven AT, Duijnhouwer AL, Eicken A, et al. Adverse outcome of coarctation stenting in patients with Turner syndrome. Catheter Cardiovasc Interv 2017;89:280-7. [Crossref] [PubMed]
- Zanjani KS, Thanopoulos BD, Peirone A, et al. Usefulness of stenting in aortic coarctation in patients with the Turner syndrome. Am J Cardiol 2010;106:1327-31. [Crossref] [PubMed]
- Fejzic Z, van Oort A. Fatal dissection of the descending aorta after implantation of a stent in a 19-year-old female with Turner's syndrome. Cardiol Young 2005;15:529-31. [Crossref] [PubMed]
- Chew JD, Hill KD, Jacobs ML, et al. Congenital Heart Surgery Outcomes in Turner Syndrome: The Society of Thoracic Surgeons Database Analysis. Ann Thorac Surg 2019;108:1430-7. [Crossref] [PubMed]
- Cramer JW, Bartz PJ, Simpson PM, et al. The spectrum of congenital heart disease and outcomes after surgical repair among children with Turner syndrome: a single-center review. Pediatr Cardiol 2014;35:253-60. [Crossref] [PubMed]
- Patel A, Hickey E, Mavroudis C, et al. Impact of noncardiac congenital and genetic abnormalities on outcomes in hypoplastic left heart syndrome. Ann Thorac Surg 2010;89:1805-13; discussion 1813-4. [Crossref] [PubMed]
- Alam S, Claxton JS, Mortillo M, et al. Thirty-Year Survival after Cardiac Surgery for Patients with Turner Syndrome. J Pediatr 2021;239:187-192.e1. [Crossref] [PubMed]
- Philip J, Gupta D, Bleiweis MS, et al. Hypoplastic left heart in Turner's syndrome: a primary indication for transplant? Cardiol Young 2018;28:458-60. [Crossref] [PubMed]
Cite this article as: Rye-Buckingham S, Furst ML. A narrative review: cardiovascular aspects of Turner syndrome in the pediatric population. Pediatr Med 2024;7:16.

