Minimal incision and less invasive techniques in congenital cardiac surgery: a narrative review
Introduction
Background
Minimally invasive cardiac surgery (MICS) allows interventions on and inside the structures of the heart and the large vessels without full opening of the chest wall (1). Its primary aim is to reduce the collateral tissue damage, and thus morbidity—without risking the completeness of the surgical repair (2). Perceived advantages of MICS are: faster and better postoperative mobilization and independence of the patients, less infections, less trauma, less blood loss and transfusions, less re-explorations (in experienced programs), less arrhythmias, less posted lactate (2). The spectrum of MICS approaches ranges from partial chest opening to keyhole surgeries performed with endoscopic and robotic manipulation and hybrid performance (3).
Rationale and knowledge gap
Minimally invasive techniques derive from adult cardiothoracic surgery and they gradually find their application in congenital cardiac surgery per ‘virtu de necessitate’ (virtue of out necessity). Whereas MICS is now a well-established modality in adults (2), minimally invasive congenital cardiac surgery (MICCS) has so far been limited due to case complexity, widespread array of ages and bodyweights of the patients, the need to often address multiple anatomical segments in a constrained operative field, limited access to peripheral cannulation for cardiopulmonary bypass (CPB), and the lack of appropriately-sized instruments (4,5). Since infants and children do not experience major additional morbidity from chest opening per se, cosmesis had remained secondary to restoration of viable haemodynamics, i.e., the completeness of the surgical procedure.
Congenital cardiac surgery still carries higher overall risk (6,7). In the words of John W. Kirklin [1907–2004], one of the founding fathers of cardiac surgery: ‘surgery is always second best. If you can do something else, it’s better. Surgery is limited’ (8). Nevertheless, surgeons have always tried to expand those limitations. Advancing initiatives such as technology improvement (miniaturization), augmented visualization (e.g., endoscopy and video-assisted surgery), emerging hybrid modality with project-based teamwork can now join together in a minimal/less invasive approach either to reduce morbidity and provide more appealing cosmesis without jeopardizing quality of the repair and patient safety. There is, however, no unified consensus on the role and approach of MICCS especially in younger children or infants undergoing complex procedures involving more than one cardiac segment. Contemporary reports of MICCS present either case series proposing a particular approach or case reports of extending the boundaries of the modality. Authors of this review—drawing privileged synergy from a coexisting pioneering MICS program for acquired heart disease at their institution—aim to present their own programmatic experience with MICCS and cross examine it with the literature.
Objective
The objective of this narrative review is to present the state of minimally invasive techniques and practices in pediatric and congenital cardiac surgery published in the contemporary medical literature; to evaluate these practices with local institutional experience; to highlight current limitations and contemporary trends and to explore potential avenues for future development. We present this article in accordance with the Narrative Review reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-23-15/rc).
Methods
The present narrative overviews landmarks of the pertinent English-language scientific literature (papers and textbooks) and combines them with the latest available [2014–2018] published outcome data from international databases (9-11). PubMed MEDLINE, Google Scholar and EACTS International Congenital Heart Database searches were performed with the keywords of ‘minimally-invasive’, ‘congenital’, ‘pediatric’, and ‘cardiothoracic surgery’. Peer-reviewed articles including original articles, systematic reviews and case reports that described minimally invasive congenital and/or pediatric cardiac surgery were selected. Articles focusing on aspects of MICS for acquired heart disease were only considered for drawing comparisons. Articles not pertaining to the topic, e.g., minimally invasive thoracic surgery, non-cardiac operations utilizing minimally-invasive perfusion techniques in the pediatric age-group were excluded (Table 1). Local experience and a perspective on program development is presented from a medium-size tertiary-care congenital cardiac centre as the authors’ viewpoint.
Table 1
| Items | Specification |
|---|---|
| Date of search | January 1 to March 15, 2023 |
| Resources searched | PubMed MEDLINE, Google Scholar, EACTS International Congenital Heart Database |
| Keywords used for search | Minimally-invasive, congenital, pediatric, and cardiothoracic surgery |
| Timeframe | Literature published until search date. EACTS Database results between 2014–2018 were accessed |
| Inclusion and exclusion criteria | Inclusion: original articles, systematic reviews and case reports in peer-reviewed journals. Language restrictions: English |
| Articles not pertaining to minimally invasive surgical procedures for congenital heart disease were excluded | |
| Selection process | L.K. and S.K.S. and reviewed all retrieved articles independently. All authors participated in the final selection of literature suitable for the review |
Discussion
The scope of MICCS
In the adult cardiothoracic practice, minimally invasive approach mostly addresses a discrete pathology, e.g., entry-point of the chest and methods append to specific valve procedures, coronary revascularization, etc. (2,12). Congenital cardiac surgery entails with a wide spectrum of anomalies where individual components often conjoin to create complex phenotypes (13). Tetralogy of Fallot (TOF), interrupted aortic arch-ventricular septal defect (VSD)-left ventricle outflow tract obstruction, etc. necessitates surgical performance in different anatomical intra- and extracardiac compartments during the same operation. Majority of the distinct congenital cardiac procedures belong to the complex, most complex classes (11,12). The complex procedures are performed in a highly constrained time-space framework (4). Historically MICCS had been introduced with some gender preference (14) for situations where less complexity and excellent outcomes allowed to consider other aspects, e.g., more appealing scars (15). Scope of indication has gradually broadened and MICCS is now accessible in the pursuit to treat more and hurt less (16). MICCS is yet to capitalize on the emerging adjuvant techniques of endoscopy, robotics and hybrid (see, later).
Preoperative preparation and intraoperative visualization
Careful patient selection for MICS/MICCS is the key in avoiding complications and conversion to full opening (2). Computed tomography (CT) angiography has become the gold standard (17) in cardiovascular imaging for assessing segmental anatomy, structures of region-of-interest as well as topography of the chest with a view of peripheral cannulation. 3D reconstruction of CT images advises on the best surgical approach and steps of the procedure. Preoperative planning by CT scan for minimally/less invasive congenital cardiac reoperations is inevitable in preventing re-entry injury (18). CT datasets along with data from other advanced imaging (3D echocardiography, magnetic resonance imaging) sources enable 3D modelling. Virtual models can be printed as 3D prototypes (physical models) and/or projected into the virtual reality as holograms (19). 3D-printed prototypes allow decision on the cardiac entry, emulation of the operation steps, so that ‘trial-and-error’ improvisation in the operating room (OR) must be avoided (20). Holographic images could be projected in augmented reality on the patient’s chest for the best entry-point (21) (Figure 1, Video 1).

Holographic images are also integrated into the OR video-recording systems [endoscopic, surgical headlight OR 360-degree cameras, transesophageal echocardiography (TEE), etc.] for reference that increases team participation in the surgical flow and serves as multiple teaching and transmission purposes (22). Detailed preoperative briefing, with written graphic notes has become a standard at the authors’ institution, that enhances communication and teamwork.
Keeping away from the midline
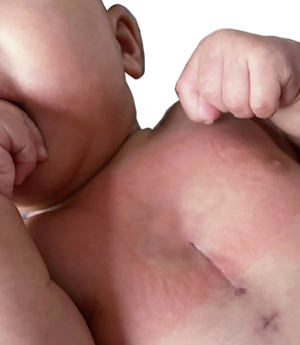
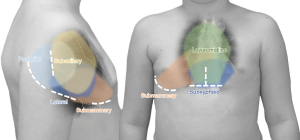
Keeping away from the midline has been proposed from the adult sternal morbidity (5). The spectrum of anomalies addressed with MICCS arrays from simple defects [atrial septal defect (ASD) sinus venosus ASD (SV-ASD), VSD, subaortic stenosis] to atrioventricular septal defect (AVSD), ToF repair and aortic and mitral valve (MV) repair, further incorporating repairs of AVSD and isolated pulmonary stenosis, extracardiac Fontan, etc. (14-16,23,24). Most reports (25-29) advise that thoracotomies provide adequate approach to more discrete (solitary) anomalies—easily addressed from the ipsilateral side [e.g., ASD, subaortic stenosis, etc. from the right; pulmonary valve, right ventricle outflow tract (RVOT), etc. from the left side]. MICCS via left anterior thoracotomy found a unique surgical (30,31) and hybrid (32,33) application for solving pulmonary valve regurgitation/RVOT problems after ToF repair. In certain anomalies, e.g., SV-ASD with partially anomalous pulmonary venous drainage, scimitar syndrome, etc., the lateral approach has gained recognition for allowing superior access to these anatomical structures (34). A limited midline approach via partial sternotomy is adequate for concurrent extra/intracardiac repairs (authors of this review have performed aortic arch repair + VSD closure from partial ministernotomy). Figure 2 illustrates minimal/less invasive incisions and their access ranges in congenital and pediatric cardiac surgery.
Lateral approach
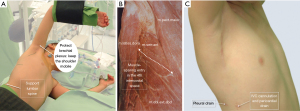
Since extrathoracic tissues are quite elastic and the layers easily slide, even small incisions (<3 cm) provide adequate view and most approaches allow muscle-sparing. The various (subaxillary, vertical, horizontal, submammary skin incisions will eventually arrive at a posterior, lateral, or anterior intercostal opening. Single lung ventilation (when double-lumen endotracheal intubation is available), retraction sutures help in gradual development of view by pulling and stabilizing the mediastinal structures towards the operator. A word of caution should be mentioned in keeping the phrenic nerve in view all the time, so its inadvertent injury and/or stretching by the stay sutures could be prevented. We found CO2-insufflation an important tool in preventing accidental air-embolism. Brachial plexus neuropraxia is prevented by proper positioning by leaving the shoulder mobile (Figure 3, Video 2). It is of note that we gradually abandon lateral decubitus position and abduction of the upper extremity as mediastinal structures move away from the operator by gravity; patients are now placed in supine position with ipsilateral side slightly elevated.
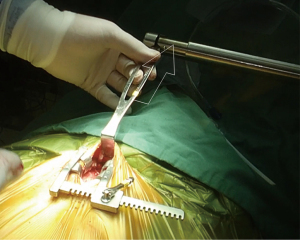
Ministernotomy
Ministernotomy exploits the pliability of sternum in younger age so that it can be suspended by a retractor mounted on an external framework (Figure 4).
A lower vertical or—less frequently—horizontal incision is followed by the separation of the rectus sheaths in the linea alba and the xyphysternum. It allows adequate view to thymus, innominate vein and arch vessels and for intracardiac procedures. Underneath, the minimal skin incision, a full sternotomy can be performed, because when the sternum is only partially incised, its lower part tends to protrude outward during healing process (35). The access permits full central cannulation for CPB. At the authors institutions, ministernotomy is routinely utilized for most extra/intracardiac repairs (ASD, VSD, ToF, arch repair, etc.) in infants and children. Its drawback that a 3-cm anterior scar still remains postoperatively (Figure 5).
Endoscopic approach
Endoscopic approach has become the gold standard in thoracic surgery and MICS, however, endoscopic and video-assisted congenital cardiac surgery lags behind despite impressive surgical series (36-38). Endoscopy has so far proven useful either in non-bypass relatively simple procedures patent ductus arteriosus ligations, vascular rings (37) and various one-compartment [ASD, VSD, MV open-heart repairs] in larger children with peripheral cannulation (36). Conventional endoscopic instrumentation lacks the dexterity required for delicate cardiac surgical procedures, and the loss of depth perception caused by two-dimensional monitors further increases operative obstacles (39). With the advent of smaller endovision systems, proper-sized instruments, endoscopy will definitely extend towards smaller children and multi-compartment lesions.
Similarly, limited reports are as yet available on robotic cardiac surgery in children (40,41). Robots operate with high-resolution, binocular, three-dimensional, magnified views of operative fields and with highly maneuverable arms handling delicate tissues and anastomoses (39) that offers a great advantage for babies.
Hybrid cardiac surgery/catheter strategies emerge as a new modality where the surgeon allowing access and performing parts of the procedure and the invasive cardiologist employing catheter interventional methods complements each other in tandem fashion (3). Availability of this modality is evolving and now includes perventricular VSD closure (42), pulmonary valve replacement (33), pulmonary artery stenting (43), pulmonary vein stenosis dilation/stenting (44), selective pulmonary artery banding with PDA stenting for hypoplastic left heart syndrome (45,46), etc. Hybrid approach can help critically-ill patients with intractable (non-cardiac) conditions, where minimalization of collateral morbidity is key (47). In a broader sense, hybrid approach illustrates progress in therapy from open access towards catheter-based procedures. Table 2 summarizes the various aspects of MICCS entry approaches.
Table 2
| Approach/incision | Suitability for | Advantage | Disadvantage | Possible complication |
|---|---|---|---|---|
| Minithoracotomy | Direct access to selected lesions; no midline scar | Mostly single compartment access; constrained operative field; difficult access for cannulation | Phrenic nerve palsy; bleeding; postoperative pain | |
| Right | Right or left-sided lesions | |||
| Subaxillary | ASD, VSD, iAVD, AoV, LVOT | |||
| Posterior | MV, ASD, pulmonary veins | |||
| Posterolateral, anterior, submammary | ASD, VSD, iAVD, AoV, LVOT, MV | |||
| Left | PDA, CoA, LAA, LV apex, pulmonary veins | |||
| Ministernotomy | Multi-compartment lesions | Intracardiac and great vessels, thymus | Midline scar | Lower sternum deformity |
| Lower partial | ASD, VSD, ToF, etc. | Multi-compartment access | ||
| Subxiphoid | VAD, PM implantation, PVR | Limited access | ||
| Endoscopy, robotics | ASD, SV-ASD, PDA | Potential benefits; miniaturization; 3D visualization | Single compartment access; limited MICCS experience | Limited MICCS experience |
| Hybrid | Stage-1 palliation for HLHS, musc-VSD, (Redo-) PVR/RVOT | Possible benefits Broadening scope | Mostly single compartment access | Stage-1: uncertain long-term outcomes, additional scar |
MICCS, minimally invasive congenital cardiac surgery; ASD, atrial septal defect; VSD, ventricular septal defect; iAVD, incomplete atrioventricular septal defect; AoV, aortic valve; LVOT, left ventricle outflow tract; MV, mitral valve; PDA, patent arterial duct; CoA, aortic coarctation; LAA, left atrial appendage; LV, left ventricle; ToF, tetralogy of Fallot; VAD, ventricular assist device; PM, pacemaker; PVR, pulmonary valve replacement; SV-ASD, sinus venosus atrial septal defect; HLHS, hypoplastic left heart syndrome; RVOT, right ventricle outflow tract.
Conversion to full chest opening
Conversion to full chest opening is rare and it is mainly associated with an unsuspected anatomical entity translating into an intractable technical problem, e.g., venous anomaly prohibiting proper drainage, restricted access/visibility, coronary anomaly. Similarly, high body mass index (BMI) can lead to an inadequate view/access that could prompt to conversion at a highest rate of 36% (48); experienced MICCS programs report around 2–5% (25,27,28). Authors of this review did not face the situation for conversion, however; intraoperative disintegration of right ventricle-to-pulmonary artery conduit necessitated extension of the incision.
Cannulation technique
The biggest challenge faced in MICCS is cannulation for CPB (49). Neck access via the internal jugular vein and common carotid artery is the favored peripheral cannulation sites in neonates and smaller children (<5–6 years or <30 kg) (50). Despite these patients are vulnerable to sustain cerebral injury (51), cervical cannulation—especially for a shorter duration of procedural CPB—balances with the flow requirements allowed by the larger vessels (52). Femoral cannulation is applicable over 15 kg (52). As most congenital procedures involve an intracardiac part, drainage of both caval territories as well as venting of the left heart is necessary. Due to the elasticity of pediatric soft tissues, however, central cannulation and venting across the operative area is usually possible (53). A useful alternative is to introduce the inferior vena cava cannula via the opening of the chest drain (Figure 3C). Since miscellaneous venous anatomies often encountered with congenital cardiac phenotypes, strategic placement of peripheral cannulation requires detailed preoperative diagnosis and planning (54). Extracorporeal circulation by peripheral cannulation drains dilated cardiac chambers and thus facilitate surgical manipulations before entering the chest and is an important adjunct in avoiding serious complication during redo chest entry (55,56).
Postoperative management and complications
MICCS is reported to have less collateral tissue damage that theoretically translates into reduced intensive care unit (ICU) and hospital length of stay (LOS) after MICCS is typically 1 day shorter than with full chest opening (16,57,58), lower use of blood products (2,10), less pain and a more expedited recovery (59). Thoracotomies, in contrast to partial sternotomy, however, involve more muscle stretching, and so, administration of local anesthetic via thoracic paravertebral block cannula is recommended (57,60). Early complications of MICCS include phrenic nerve palsy associated with right thoracotomy, especially in adolescents with deeper mediastinal structure and more traction on the pericardium (61). Submammary and anterior thoracotomy incision may affect late breast development (62).
Table 3 summarizes the various aspects and parameters related to MICCS. Reports in the scientific literature are difficult to extrapolate and unify for the heterogeneity of patient populations, diagnoses, procedural protocols in different institutional settings and individual surgeons’ and teams’ preferences (67).
Table 3
| Parameter | Observation and comment | References |
|---|---|---|
| Optimal age, weight | Mini-sternotomy: from 2 weeks to prepubescents; 3.5 to 20 kg | (63) |
| Lateral approach: from 6–9 months; from 6 kg (optimal weight around 10–15 kg) | (23-28) | |
| Peripheral cannulation | Recommended above 15 kg bodyweight | (14,50,52,64) |
| Procedural length, CPB, AoCC | MICCS has longer/similar procedural length | (15,58,65) |
| No significant difference in CPB, AoCC durations from full opening | (26-28,63,64) | |
| Conversion rate to full opening | 0.1–0.2% | (14,15,25,26,28) |
| IPPV | Typically, less than 6 h | (14,15,23-28,63,64) |
| Analgesia | MICCS is associated with reduced analgesia need | (60,66) |
| Blood conservation | Reduced utilization of transfusions | (2,10) |
| Complication | Overall minimal complication rate with MICCS: (I) permanent AV block, 0.1–1%; (II) pneumothorax, 0.1–0.9%; (III) pericardial/pleural effusion, 0.1–0.9%; (IV) postoperative bleeding requiring exploration, 0.1–0.6%; (V) reoperation for residual defects, 0.2–0.5% | (14,15,23-28,57,63,64,66) |
| LOS | MICCS is associated with one day shorter LOS than full opening | (16,57,58) |
| Implications on costs | Difficult to quantify for inhomogeneity and lack of data. MICCS may theoretically reduce costs | (57,67) |
MICCS, minimally invasive congenital cardiac surgery; CPB, cardiopulmonary bypass; AoCC, aortic cross-clamp time; IPPV, intermittent positive pressure ventilation; AV, atrioventricular; LOS, length of stay.
MICCS program building
Our cardiac surgical heritage stands on the shoulder of pioneering giants who had had the courage and stamina to implement new techniques and modalities, sometimes, by trespassing established boundaries (68). Such an individual achievement and even bravado is no longer possible as clinical practice improvement is imprisoned in multilevel regulations (69-71). It is, therefore, an imperative that adult and congenital surgeons learn from one another (72). Table 4 summarizes differences between MICS for acquired heart disease and minimal invasive and less invasive techniques in adult and pediatric age congenital cardiac surgery by institutional experience. In conclusion, a synergy is observed between the MICS (i.e., acquired heart disease) and MICCS (congenital anomalies) teams at the authors’ institutions, where the ‘adult’ team contributes with the experience from standardized, high-volume MICS procedures whereas the pediatric team capitalizes on the experience with pathological and physical subtleties. Cooperation must extend to the Heart Team in view of hybrid approaches and—in another dimension—to the multidisciplinary team to deal with comorbidities, sociopsychological aspects of especially adult congenital heart disease (ACHD) patients.
Table 4
| Aspects | MICS for acquired heart disease | ACHD | PCHD | Institutional experience |
|---|---|---|---|---|
| Presentation | Adult age and parameters | Widespread age/bodyweight range | ACHD capitalizes on adult MICS techniques | |
| Pathologies | Single compartment pathologies (e.g., ischaemic heart disease, valve problems, ASD); high case volume; repetition is common | Often multi-compartment scenarios, high variety; case repetition is rare | ACHD is suitable for standardization, whereas PCHD mostly requires individual planning | |
| Procedures | Adult MICS techniques applicable (e.g., valve repair/replacement) | Wide variety of individual procedures; extracardiac/intracardiac procedures (e.g., aortic arch repair and intracardiac repair) | MICCS standardization for PCHD in progress | |
| Cannulation for CPB | Peripheral access | Central access; jugular access in selected cases | Miniaturization could lower the bodyweight for peripheral cannulation in pediatric MICCS | |
| Perioperative setting | Heart-team | Close cooperation of the multidisciplinary CHD team with adult MICS team is mandatory | ||
MICS, minimally invasive cardiac surgery; CHD, congenital heart disease; ACHD, adult congenital heart disease; PCHD, pediatric age congenital heart disease; ASD, atrial septal defect; CPB, cardiopulmonary bypass; MICCS, minimally invasive congenital cardiac surgery.
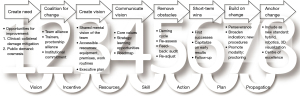
As mentioned, minimally invasive cardiac surgical techniques mostly derive from the adult practice and their implementation for the widespread range of the paediatric cardiac population is only possible as a programmatic change. Multidisciplinary communication provides flexible learning opportunities (20). The steep learning curve (73) and consistently maintained results both at individual and team level mandates proper mentoring, readily available at larger centers (74,75). Proctorship by an established minimally invasive cardiac program is strongly recommended (2). As for a new clinical modality, strong institutional commitment is essential for accessible resources: equipment, i.e., special instruments and toolkit, dedicated premises (e.g., hybrid OR), provision of working routines. Commitment of the entire multidisciplinary team creates alliance that is a key for development, preserving high standards along the entire continuum-of-care. Team empowerment also introduces the best motivation for change which itself is inevitable (76). Figure 6 illustrates the chain of change adapted for a holistic MICCS scenario.
Future trends
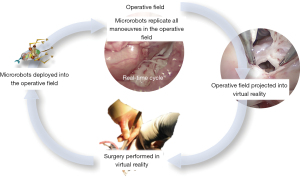
It is expected that development of the art of MICCS will extend into two directions: (I) indication for minimally invasive methods will range to complex neonates. The very instability of these fragile patients will invoke new and less invasive approaches. A view on the history of pediatric cardiac surgery demonstrates that multidisciplinary synergy lessens risk, e.g., in the case of Rashkind-septostomy instead of Blalock-Hanlon septectomy (77), or more recently, PDA or RVOT stenting, instead of Blalock-Taussig-shunt (78). For complete complex neonatal repairs, a similar trend is expected that will capitalize on the advances of all related disciplines, and perhaps, more on the non-related ones (79). Nevertheless, there are multiple barriers of space- and time-constraints still need to conquered (57). (II) Reoperations could also be a promoter of MICCS involving hybrid techniques, advances in translational research and biofabrication that respectively reduce procedural risk (individual patient benefit) and to save the burden of multiple open replacements of traditional biologic tissue valves and conduits (public health benefit) (80,81). Improved preoperative imaging, emulation techniques complemented with intraoperative 3D visualization (e.g., virtual/augmented reality) and manipulation (e.g., robotics) will broaden the scope of indication for MICCS (82). Microrobots show the potential to revolutionize medicine and surgery (83). Magnetically powered microrobots have already been utilized in selected indications in interventional cardiology and cardiac surgery (84). Cutting edge research in this field aims for lightweight, untethered, wirelessly controllable and powered devices with modular arms that could be deployed into the operative field to perform new procedures never before possible (85,86). Figure 7 illustrates the authors’ vision of performing intracardiac procedures on beating heart without CPB.
Surgery is an essentially multifactorial, multitasking activity, where time-information constraints are prone to cause individual and team cognitive dissonance and distress (87). Change is always stressful (76). MICCS is to be complemented by the accomplishments of the allied and non-allied disciplines, e.g., information technology, material science; these should assist in reducing the risk for patients and the stress for the personnel. Congenital cardiac surgery sprouted off from the trunk of surgery via cardiothoracic surgery some 60 years ago. The first congenital surgeons spent considerable time in the surgical laboratory and pathology museum. The next generation of surgeons may well come from scientific labs and/or from gaming platforms.
Strengths and limitations
MICCS is a strengthening and broadening modality in an evolving technical and intellectual multidisciplinary environment. MICCS holds the promise to replicate MICS experience in adults in reducing morbidity, possibly decreasing medical costs by the avoidance of complications, LOS, and resource utilization. Thus, MICCS has its strengths in communication/cooperation with its adult counterparts. That synergy could well open up this market for accelerated innovation in miniaturization from the industry.
Congenital cardiac surgery—in comparison with surgery for acquired heart disease—features widespread presentations and pathologies that make MICCS procedural standardization difficult. At present, technical limitations persist to extend MICCS to neonatal, infant multicompartment procedures and peripheral cannulation. As constant repetition of standard MICCS is limited, proficiency may take a longer time to acquire. Technical difficulties in the learning phase may associate with longer CPB and myocardial ischaemia durations. Lack of access to proctorship also hamper development. Diverse institutional and technical background and financial abilities in which individual surgical teams operate result in that no unified MICCS approach is currently adopted. The phenomenon surfaces as publication bias which makes evaluation of MICCS’ current state uncertain.
Conclusions
This review only offers a snapshot on MICCS as a developing modality. It may seem that cosmesis drives MICS/MICCS efforts, however, it is the reduction of collateral trauma, and morbidity that the modality primarily targets. MICCS offers an alternative to conventional open surgery in an environment where not only survival matters but also quality-of-life and avoidance of complications. MICCS relies on careful personalized planning and execution throughout the entire continuum-of-care, and so, it requires full multidisciplinary team buy-in. Indication of MICCS gradually broadens, however, it is yet to reach complex neonatal/infant procedures involving multiple operative compartments. Progress in MICCS not only mandates a development of new multimodality imaging-emulation and manipulation techniques, but new materials and equipment, and perhaps a change of mindset, too.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-23-15/rc
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-23-15/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-23-15/coif). L.K. serves as an unpaid editorial board member of Pediatric Medicine from July 2022 to June 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The guardians of the patients on the figures and videos gave their written consent to the publication of the photographs and videos.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dieberg G, Smart NA, King N. Minimally invasive cardiac surgery: A systematic review and meta-analysis. Int J Cardiol 2016;223:554-60. [Crossref] [PubMed]
- Kofidis T. Minimally Invasive Cardiac Surgery. A Practical Guide. CRC Press: Boca Raton FL, USA, 2021.
- Bacha E, Kalfa D. Minimally invasive paediatric cardiac surgery. Nat Rev Cardiol 2014;11:24-34. [Crossref] [PubMed]
- del Nido PJ. Minimal incision congenital cardiac surgery. Semin Thorac Cardiovasc Surg 2007;19:319-24. [Crossref] [PubMed]
- Alsarraj MK, Nellis JR, Vekstein AM, et al. Borrowing from Adult Cardiac Surgeons-Bringing Congenital Heart Surgery Up to Speed in the Minimally Invasive Era. Innovations (Phila) 2020;15:101-5. [Crossref] [PubMed]
- Coulson JD, Seddon MR, Readdy WF. Advancing safety in pediatric cardiology—approaches developed in aviation. Congen Cardiol Today 2008;6:1-10.
- Austin JM, Derk JM, Pronovost PJ. An analysis of publicly reported pediatric heart surgery data and patient mortality implications. J Hosp Manag Health Policy 2018;2:50. [Crossref]
- John W. Kirklin Quotes. Available online: https://www.azquotes.com/author/29196-John_W_Kirklin, accessed on 9 May 2022.
- St Louis JD, Deng L, Covington C, et al. The World Society for Pediatric and Congenital Heart Surgery: 2021 Update of the World Database for Pediatric and Congenital Heart Surgery. World J Pediatr Congenit Heart Surg 2022;13:137-45. [Crossref] [PubMed]
- World Database For Pediatric And Congenital Heart Surgery. Available online: https://www.wspchs.org/world-database/world-database-for-pediatric-and-congenital-heart-surgery, accessed on 30 December 2022.
- Jacobs JP, Mayer JE Jr, Pasquali SK, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2019 Update on Outcomes and Quality. Ann Thorac Surg 2019;107:691-704. [Crossref] [PubMed]
- Iribarne A, Easterwood R, Chan EY, et al. The golden age of minimally invasive cardiothoracic surgery: current and future perspectives. Future Cardiol 2011;7:333-46. [Crossref] [PubMed]
- Tynan MJ, Becker AE, Macartney FJ, et al. Nomenclature and classification of congenital heart disease. Br Heart J 1979;41:544-53. [Crossref] [PubMed]
- Vida VL, Padalino MA, Boccuzzo G, et al. Minimally invasive operation for congenital heart disease: a sex-differentiated approach. J Thorac Cardiovasc Surg 2009;138:933-6. [Crossref] [PubMed]
- Schreiber C, Bleiziffer S, Kostolny M, et al. Minimally invasive midaxillary muscle sparing thoracotomy for atrial septal defect closure in prepubescent patients. Ann Thorac Surg 2005;80:673-6. [Crossref] [PubMed]
- Karangelis D, Androutsopoulou V, Tzifa A, et al. Minimally invasive cardiac surgery: in the pursuit to treat more and hurt less. J Thorac Dis 2021;13:6209-13. [Crossref] [PubMed]
- Aviram G, Sharony R, Kramer A, et al. Modification of surgical planning based on cardiac multidetector computed tomography in reoperative heart surgery. Ann Thorac Surg 2005;79:589-95. [Crossref] [PubMed]
- Imran Hamid U, Digney R, Soo L, et al. Incidence and outcome of re-entry injury in redo cardiac surgery: benefits of preoperative planning. Eur J Cardiothorac Surg 2015;47:819-23. [Crossref] [PubMed]
- Goo HW, Park SJ, Yoo SJ. Advanced Medical Use of Three-Dimensional Imaging in Congenital Heart Disease: Augmented Reality, Mixed Reality, Virtual Reality, and Three-Dimensional Printing. Korean J Radiol 2020;21:133-45. [Crossref] [PubMed]
- Kiraly L, Shah NC, Abdullah O, et al. Three-Dimensional Virtual and Printed Prototypes in Complex Congenital and Pediatric Cardiac Surgery-A Multidisciplinary Team-Learning Experience. Biomolecules 2021;11:1703. [Crossref] [PubMed]
- Brun H, Bugge RAB, Suther LKR, et al. Mixed reality holograms for heart surgery planning: first user experience in congenital heart disease. Eur Heart J Cardiovasc Imaging 2019;20:883-8. [Crossref] [PubMed]
- Aye WMM, Kofidis T, Kiraly L. Intraoperative video recording using 5 different camera systems for an educational purpose. Accepted abstract for 23rd ISMICS Annual Scientific Meeting, Boston, 31 May- 3 June 2023.
- Lee T, Weiss AJ, Williams EE, et al. The Right Axillary Incision: A Potential New Standard of Care for Selected Congenital Heart Surgery. Semin Thorac Cardiovasc Surg 2018;30:310-6. [Crossref] [PubMed]
- Nguyen K, Chin C, Lee DS, et al. The axillary incision: a cosmetic approach in congenital cardiac surgery. J Thorac Cardiovasc Surg 2007;134:1358-60. [Crossref] [PubMed]
- Yang X, Hu Y, Dong J, et al. Rightvertical axillary incision for atrial septal defect: a propensity score matched study. J Cardiothorac Surg 2022;17:256. [Crossref] [PubMed]
- Yang X, Wang D, Wu Q. Repair of atrial septal defect through a minimal right vertical infra-axillary thoracotomy in a beating heart. Ann Thorac Surg 2001;71:2053-4. [Crossref] [PubMed]
- Wang Q, Li Q, Zhang J, et al. Ventricular septal defects closure using a minimal right vertical infraaxillary thoracotomy: seven-year experience in 274 patients. Ann Thorac Surg 2010;89:552-5. [Crossref] [PubMed]
- An K, Li S, Yan J, et al. Minimal Right Vertical Infra-axillary Incision for Repair of Congenital Heart Defects. Ann Thorac Surg 2022;113:896-902. [Crossref] [PubMed]
- Mashadi AH, Said SM. Resection of Cor Triatriatum Dexter via Vertical Right Axillary Thoracotomy in an Infant. Available online:
10.25373/ctsnet.21916371.v1 , accessed on 15 January 2023.10.25373/ctsnet.21916371.v1 - Ramman TR, Chowdhuri KR, Raja N, et al. Pulmonary Valve Replacement in Repaired Tetralogy of Fallot Through Limited Left Anterolateral Thoracotomy: An Alternative to Repeat Sternotomy. World J Pediatr Congenit Heart Surg 2020;11:346-9. [Crossref] [PubMed]
- Henaine R, Yoshimura N, Di Filippo S, et al. Pulmonary valve replacement in repaired tetralogy of Fallot by left thoracotomy avoid ascending aorta injury. J Thorac Cardiovasc Surg 2011;141:590-2. [Crossref] [PubMed]
- Carr K, Nijres BM, Windsor JJ, et al. Single-Center Experience of Hybrid Pulmonary Valve Replacement Using Left Anterior Thoracotomy With Pulmonary Artery Plication in Patients With Large Right Ventricular Outflow Tract. J Am Heart Assoc 2022;11:e026517. [Crossref] [PubMed]
- Suleiman T, Kavinsky CJ, Skerritt C, et al. Recent Development in Pulmonary Valve Replacement after Tetralogy of Fallot Repair: The Emergence of Hybrid Approaches. Front Surg 2015;2:22. [Crossref] [PubMed]
- Diliz-Nava HS, López Terrazas JH, Palacios Macedo-Quenot AJ, et al. The Lugones Procedure for Scimitar Syndrome Repair Through Right Axillary Thoracotomy: Not Only a Cosmetically Superior Approach. World J Pediatr Congenit Heart Surg 2022;13:777-81. [Crossref] [PubMed]
- Breatnach CR, McGuinness J, Ng LY, et al. Procedural technique for hybrid pulmonary valve replacement in infants and small children. Eur J Cardiothorac Surg 2021;59:823-30. [Crossref] [PubMed]
- Cheng YG, Wang YJ, Zhang Q, et al. Thoracoscopic cardiac surgical procedures: a report of 674 cases. Zhonghua Wai Ke Za Zhi 2007;45:1521-3. [PubMed]
- Villa E, Vanden Eynden F, Le Bret E, et al. Paediatric video-assisted thoracoscopic clipping of patent ductus arteriosus: experience in more than 700 cases. Eur J Cardiothorac Surg 2004;25:387-93. [Crossref] [PubMed]
- Burkhart HM, Suri RM. Minimally invasive video assisted surgical closure of secundum atrial septal defect. Ann Cardiothorac Surg 2017;6:60-3. [Crossref] [PubMed]
- Ishikawa N, Watanabe G. Robot-assisted cardiac surgery. Ann Thorac Cardiovasc Surg 2015;21:322-8. [Crossref] [PubMed]
- Raju V, Burkhart HM, Cetta F Jr, et al. Successful robot-assisted repair of congenital mitral valve regurgitation. Ann Thorac Surg 2014;98:1085-7. [Crossref] [PubMed]
- Gao C, Yang M, Wang G, et al. Totally endoscopic robotic atrial septal defect repair on the beating heart. Heart Surg Forum 2010;13:E155-8. [Crossref] [PubMed]
- Schreiber C, Vogt M, Kühn A, et al. Periventricular closure of a perimembranous VSD: treatment option in selected patients. Thorac Cardiovasc Surg 2012;60:78-80. [Crossref] [PubMed]
- Menon SC, Cetta F, Dearani JA, et al. Hybrid intraoperative pulmonary artery stent placement for congenital heart disease. Am J Cardiol 2008;102:1737-41. [Crossref] [PubMed]
- Yoon JK, Kim GB, Song MK, et al. Hybrid Pulmonary Vein Stenting in Infants with Refractory to Surgical Pulmonary Vein Stenosis Repair. Pediatr Cardiol 2018;39:1642-9. [Crossref] [PubMed]
- Galantowicz M, Cheatham JP, Phillips A, et al. Hybrid approach for hypoplastic left heart syndrome: intermediate results after the learning curve. Ann Thorac Surg 2008;85:2063-70; discussion 2070-1. [Crossref] [PubMed]
- Ceneri NM, Desai MH, Tongut A, et al. Hybrid strategy in neonates with ductal-dependent systemic circulation and multiple risk factors. J Thorac Cardiovasc Surg 2022;164:1291-1303.e6. [Crossref] [PubMed]
- Haponiuk I, Chojnicki M, Jaworski R, et al. Alternative hybrid and staged interventional treatment of congenital heart defects in critically ill children with complex and non-cardiac problems. Wideochir Inne Tech Maloinwazyjne 2015;10:244-56. [Crossref] [PubMed]
- Nellis JR, Daneshmand MA, Gaca JG, et al. A single center experience with minimally invasive approaches in congenital cardiac surgery. J Thorac Dis 2021;13:5818-25. [Crossref] [PubMed]
- Garg P, Bishnoi AK, Lakhia K, et al. Cervical Cannulation for Surgical Repair of Congenital Cardiac Defects in Infants and Small Children. Braz J Cardiovasc Surg 2017;32:111-7. [Crossref] [PubMed]
- Brown G, Moynihan KM, Deatrick KB, et al. Extracorporeal Life Support Organization (ELSO): Guidelines for Pediatric Cardiac Failure. ASAIO J 2021;67:463-75. Erratum in: ASAIO J 2022;68:e129. [Crossref] [PubMed]
- Werho DK, Pasquali SK, Yu S, et al. Epidemiology of Stroke in Pediatric Cardiac Surgical Patients Supported With Extracorporeal Membrane Oxygenation. Ann Thorac Surg 2015;100:1751-7. [Crossref] [PubMed]
- Johnson K, Jarboe MD, Mychaliska GB, et al. Is there a best approach for extracorporeal life support cannulation: a review of the extracorporeal life support organization. J Pediatr Surg 2018;53:1301-4. [Crossref] [PubMed]
- Kale SB, Ramalingam S. Minimally Invasive Cardiac Surgery Without Peripheral Cannulation: A Single Centre Experience. Heart Lung Circ 2019;28:1728-34. [Crossref] [PubMed]
- Perry T, Brown T, Misfeldt A, et al. Extracorporeal Membrane Oxygenation in Congenital Heart Disease. Children (Basel) 2022;9:380. [Crossref] [PubMed]
- Singh AK, Stearns G, Maslow A, et al. Redo sternotomy for cardiac reoperations using peripheral heparin-bonded cardiopulmonary bypass circuits without systemic heparinization: technique and results. J Cardiothorac Vasc Anesth 2011;25:347-52. [Crossref] [PubMed]
- Luciani N, Anselmi A, De Geest R, et al. Extracorporeal circulation by peripheral cannulation before redo sternotomy: indications and results. J Thorac Cardiovasc Surg 2008;136:572-7. [Crossref] [PubMed]
- Nakanishi K, Matsushita S, Kawasaki S, et al. Safety advantage of modified minimally invasive cardiac surgery for pediatric patients. Pediatr Cardiol 2013;34:525-9. [Crossref] [PubMed]
- Luo H, Wang J, Qiao C, et al. Evaluation of different minimally invasive techniques in the surgical treatment of atrial septal defect. J Thorac Cardiovasc Surg 2014;148:188-93. [Crossref] [PubMed]
- Lamelas J, Chen PC, Loor G, et al. Successful Use of Sternal-Sparing Minimally Invasive Surgery for Proximal Ascending Aortic Pathology. Ann Thorac Surg 2018;106:742-8. [Crossref] [PubMed]
- Korsik E, Meineri M, Zakhary WZA, et al. Persistent and acute postoperative pain after cardiac surgery with anterolateral thoracotomy or median sternotomy: A prospective observational study. J Clin Anesth 2022;77:110577. [Crossref] [PubMed]
- Helps BA, Ross-Russell RI, Dicks-Mireaux C, et al. Phrenic nerve damage via a right thoracotomy in older children with secundum ASD. Ann Thorac Surg 1993;56:328-30. [Crossref] [PubMed]
- Bleiziffer S, Schreiber C, Burgkart R, et al. The influence of right anterolateral thoracotomy in prepubescent female patients on late breast development and on the incidence of scoliosis. J Thorac Cardiovasc Surg 2004;127:1474-80. [Crossref] [PubMed]
- Nicholson IA, Bichell DP, Bacha EA, et al. Minimal sternotomy approach for congenital heart operations. Ann Thorac Surg 2001;71:469-72. [Crossref] [PubMed]
- Guariento A, Doulamis IP, Blitzer D, et al. Minimally Invasive Congenital Cardiac Surgery: A Large Volume European Experience. Congenital Heart Disease 2020;15:127-39. [Crossref]
- Hu CX, Tan J, Chen S, et al. Comparison of clinical outcomes and postoperative recovery between two open heart surgeries: minimally invasive right subaxillary vertical thoracomy and traditional median sternotomy. Asian Pac J Trop Med 2014;7:625-9. [Crossref] [PubMed]
- Mihályi S, Király L, Prodán Z, et al. Right subaxillary and posterolateral thoracotomy for open repair of congenital heart defects. Orv Hetil 2005;146:299-304. [PubMed]
- Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421-6; discussion 427-8. [Crossref] [PubMed]
- Westaby S, Bosher C. Landmarks in cardiac surgery. London: Informa Helathcare, 2009.
- Miller GW. King of hearts. New York: Crown Publishers, 2000.
- Ruhlman M. Walk on water. The miracle of saving Children’s lives. Penguin Books, NYC, 2004.
- Espuny Pujol F, Pagel C, Brown KL, et al. Linkage of National Congenital Heart Disease Audit data to hospital, critical care and mortality national data sets to enable research focused on quality improvement. BMJ Open 2022;12:e057343. [Crossref] [PubMed]
- Stephens E, Dearani JA, McCarthy PM, et al. What Can Adult and Congenital Surgeons Learn From One Another–Parts I and II. May 2021. Accessed on 10 January 2023. doi:
10.25373/ctsnet.14535765 .10.25373/ctsnet.14535765 - Holzhey DM, Seeburger J, Misfeld M, et al. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013;128:483-91. [Crossref] [PubMed]
- Cohen MS, Jacobs JP, Quintessenza JA, et al. Mentorship, learning curves, and balance. Cardiol Young 2007;17:164-74. [Crossref] [PubMed]
- Mussa S, Drury NE, Stickley J, et al. Mentoring new surgeons: can we avoid the learning curve?†. Eur J Cardiothorac Surg 2017;51:291-9. [PubMed]
- Kotter JP. Leading the change. Harvard Business Review Press, Boston, 2012.
- Wernovsky G, Mayer JE Jr, Jonas RA, et al. Factors influencing early and late outcome of the arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg 1995;109:289-301; discussion 301-2. [Crossref] [PubMed]
- Van Arsdell GS, Levi DS. Neonatal Tetralogy Staged Versus Complete Repair: Is it Time to Rethink Neonatal Tetralogy? J Am Coll Cardiol 2019;74:1580-1. [Crossref] [PubMed]
- Castaneda AR, Mayer JE Jr, Jonas RA, et al. The neonate with critical congenital heart disease: repair: a surgical challenge. J Thorac Cardiovasc Surg 1989;98:869-75. [Crossref] [PubMed]
- Galantowicz M. Encouraging six-month results with Gore novel biosynthetic tissue valve. Available online: https://www.prnewswire.com/news-releases/w-l-gore--associates-announces-encouraging-six-month-results-with-its-novel-biosynthetic-tissue-valve-301180050.html, retrieved 15 December 2020.
- Kiraly L, Vijayavenkataraman S. Biofabrication in Congenital Cardiac Surgery: A Plea from the Operating Theatre, Promise from Science. Micromachines (Basel) 2021;12:332. [Crossref] [PubMed]
- Del Nido PJ. Minimally Invasive Cardiac Surgical Procedures in Children. Innovations (Phila) 2020;15:95-8. [Crossref] [PubMed]
- Nelson BJ, Kaliakatsos IK, Abbott JJ. Microrobots for minimally invasive medicine. Annu Rev Biomed Eng 2010;12:55-85. [Crossref] [PubMed]
- Chautems C, Zeydan B, Charreyron S, et al. Magnetically powered microrobots: a medical revolution underway? Eur J Cardiothorac Surg 2017;51:405-7. [Crossref] [PubMed]
- Ceylan H, Yasa IC, Kilic U, et al. Translational prospects of untethered medical microrobots. Prog Biomed Eng 2019;1:012002. [Crossref]
- Hurley J. Building robots ready for cutting edge of medicine. The Times, Business (printed version):43, Wednesday, 16 August 2017.
- Shultz TR, Lepper MR. Cognitive dissonance reduction as constraint satisfaction. Psychol Rev 1996;103:219-40. [Crossref] [PubMed]
Cite this article as: Kiraly L, Aye WMM, Subbian SK, Kofidis T, Al Hakami A. Minimal incision and less invasive techniques in congenital cardiac surgery: a narrative review. Pediatr Med 2024;7:14.