
Recognising and managing sepsis in children’s emergency care: a clinical practice review
Introduction
Paediatric sepsis imposes a significant global burden on the health of children, with outcomes for children dependent on timely recognition and management (1,2). The majority of patients with paediatric sepsis will seek support in emergency care settings on first presentation, but minor febrile illnesses among paediatric patients represent a significant proportion of visits to the emergency department (ED) (2,3). Trying to discern these benign cases from the significantly smaller number demonstrating true sepsis remains an ongoing challenge (3). Defining sepsis also remains challenging, with differing consensus opinions on the applicability and relevance of criteria to children (4). This is further complicated by screening and decision tools that are currently overly sensitive and insufficiently specific.
The Surviving Sepsis Campaign (SSC) has focused on standardising the recognition and management of sepsis in children (2). This article, informed by SSC recommendations and more recent emerging evidence, explores the current and future landscape of recognising and managing sepsis in the emergency care of children (Figure 1).
Compared to previous clinical review articles on sepsis, this article benefits from synthesising the recently published Phoenix criteria for paediatric sepsis and septic shock, which were released after the SSC recommendations (2,5). It also considers the impact of digital technologies and artificial intelligence (AI) on children’s emergency care. Moreover, most of the global mortality in sepsis occurs in low- and middle-income countries (LMICs), and this review considers how recent advances in recognising and managing sepsis relate to healthcare inequities (6-8).
Defining sepsis
There is a significant discrepancy in administrative, clinical, and research definitions of sepsis, which are continuously evolving (Table 1) (2,9).
Table 1
| Definition | IPSCC 2005 | Sepsis-3 criteria 2016 | Phoenix criteria for pediatric sepsis 2024 |
|---|---|---|---|
| Sepsis | SIRS in the presence of suspected or proven infection | SOFA score ≥2 points + infection | Phoenix sepsis score ≥2 points + suspected infection |
| Severe sepsis | Sepsis + cardiovascular organ dysfunction or acute respiratory distress syndrome or two or more other organ dysfunctions | N/A | N/A |
| Septic shock | Sepsis and cardiovascular organ dysfunction | Vasopressors + serum lactate level >2 mmol/L | Sepsis with ≥1 cardiovascular point(s) |
IPSCC, International Pediatric Sepsis Consensus Conference; N/A, not applicable; SIRS, systemic inflammatory response syndrome; SOFA, Sequential (Sepsis-Related) Organ Failure Assessment.
Emphasising the need for a sensitive definition which facilitates early recognition of sepsis, in 2005, the International Paediatric Sepsis Consensus Conference (IPSCC) proposed criteria for sepsis, which are still widely used (10). This involves characterising sepsis as a suspected or confirmed infection in the presence of systemic inflammatory response syndrome (SIRS), which can be identified by abnormal physiological observations (fever, tachycardia, tachypnoea) (10). The sensitivity of the IPSCC criteria is at the expense of its specificity, as the criteria label many children with mild illness severity as having sepsis, which may lead to alarm fatigue; this also does not reliably identify children at risk of worse outcomes (11).
Sepsis criteria were updated in 2016 in the form of the sepsis-3 criteria (12). These identified sepsis using an increase in the Sequential Organ Failure Assessment (SOFA) score of at least two points in patients with suspected infection (12). However, the sepsis-3 criteria have not been validated or adapted for children and are therefore not widely used in children’s emergency care (4,11). Moreover, neither the IPSCC nor the sepsis-3 task forces included any experts from LMICs, and there may be challenges in applying these definitions to resource-limited settings (11). The perceived usefulness of the IPSCC and sepsis-3 definitions were evaluated in an international survey published in 2022, which received 2,835 respondents, 48% from upper-middle-income countries (UMICs), 38% from high-income countries (HICs), and 14% from low or LMICs (11). Although this survey included LMICs, they were still underrepresented (11). Overall, neither of the definitions was considered to be useful (defined as agree or strongly agree) by more than 50% of respondents (11). Respondents concurred that future definitions should amend the concept of sepsis as it relates to children without organ dysfunction in a hospital context, and that criteria should be applicable or adaptable to resource-limited settings (11).
More recently, in 2024, the Phoenix criteria for paediatric sepsis and septic shock were developed (5). These criteria were created following international surveys, analyses of over three million children’s ED attendances, and a modified Delphi process (5,13). This has the advantage over other criteria of being derived and validated in both high and limited-resource settings and is, therefore, more likely to have global applicability (14). The Phoenix criteria consider the child’s age and combine physiological observations with relevant biomarkers and treatment requirements (5). The new definition for sepsis is “an infection with life-threatening organ dysfunction”, which involves four organ system variables that affect the score: respiratory, cardiovascular, coagulation, and neurological variables (5). Although promising, there are concerns that some universally available physical findings, such as capillary refill, are not included in the criteria, which could limit its efficacy in resource-limited settings (14). However, overall, this criterion is a better predictor of life-threatening organ dysfunction, and in the future, it may be more widely adopted into clinical guidelines; currently, however, this criterion is not yet widely used (14).
Screening for sepsis
Screening
Identifying bacterial infection and its clinical syndrome, sepsis, has long posed a diagnostic challenge in paediatric emergency care (15). Given the potential sequelae of the disease, investigating feverish children for significant bacterial infection has led to the development of a common vernacular: the terms “Septic screen” and “Septic screening”. However, these terms may be unhelpful to clinicians, patients, and their families, and it has been suggested that using such terms should be phased out of the paediatric ED (16).
Validated tools exist to aid in the identification of children with significant bacterial infections and sepsis (5,17,18). These, however, often require the use of biomarkers. Routine phlebotomy is not commonplace in paediatric ED due to the logistical considerations of obtaining blood samples in children. Therefore, the emergency clinician’s diagnostic challenge lies in identifying which children will benefit from such tools and deciding when this application is appropriate. The literature is highly heterogeneous when discussing the best approach to identifying sepsis in the paediatric ED (19). Given modern convergence on outcome-focused research, a high volume of work is currently being published in the field (19). This includes potential technologies such as ribonucleic acid (RNA) expression profiling and point-of-care pathogen identification within the ED.
Age-bound physiological observation thresholds are set out in the National Institute for Health and Care Excellence (NICE) guidance, a United Kingdom (UK) body for standardisation of guidelines, for identifying children at “high-risk” of significant bacterial infection (3). However, these have been shown to be conservative, with up to 28.7% of children in some age groups being identified as high-risk using these values (20). In fact, there is a significant body of evidence to suggest that commonly used values are overly conservative and may represent normal physiological parameters (21-23). Therefore, current practice may lead to over-investigation resultant of using these values. This may lead to overtreatment of children who are unlikely to develop sepsis, and alarm fatigue, which may lead to complacency in investigating these children (20,24).
Decision tools in recognising sepsis
When children present to EDs, trying to discern the septic patient amongst the plethora of febrile children remains a considerable challenge. Thus, multiple paediatric clinical decision support (CDS) tools are available to support practice, of which clinical guidelines fall into this broad category (25). CDS tools need to be sensitive enough to enable clinicians to recognise the conditions promptly but specific enough so as not to over-treat patients, causing potential harm whilst wasting clinical and financial resources (25,26).
The NICE sepsis guidelines are widely accepted within the UK to guide decisions in recognising and managing paediatric sepsis and form the basis of many local unit protocols (3). Guidance stratifies patients into different risk categories based upon expanded SIRS criteria (3). However, they have not been validated, and several studies have demonstrated their poor discrimination in recognising serious bacterial infections, leading to potential excess antibiotic use and unnecessary admissions (27-29).
For example, the utility of the NICE sepsis guidelines was recently explored in an at-risk population of children with community-acquired pneumonia (CAP) (29). This study was a secondary analysis of the “Effect of Amoxicillin Dose and Treatment Duration on the Need for Antibiotic Re-treatment in Children With Community-Acquired Pneumonia” study, which explored the use of oral antibiotics for children with CAP being discharged from an ED within 48 hours (29,30). The study examined whether children with low-risk pneumonia (able to be discharged with oral antibiotics from an ED) had high-risk sepsis criteria based on NICE sepsis guidelines (29). The authors assumed that there was no organ dysfunction in the children safely discharged home and therefore considered them not to have sepsis according to the recently released Phoenix criteria (13). This study observed 318/591 false positives for high-risk sepsis, giving the NICE guidelines a specificity of 46% and demonstrating poor utility (29).
A recent systematic review provided an overview of evaluated sepsis diagnostic tools over the last two decades (19). Biomarkers in isolation had been the most extensively studied (44% of studies), followed by using clinical variables or parameters during clinician assessments (22%) or a combination of the two (19%) (19). In recent years, there has been an increase of studies reviewing the combined addition of electronic alerts (19). However, due to the significant heterogeneity in using different reference standards for sepsis definitions, this limited ongoing meta-analysis and further data interrogation to easily compare the performance of the different tools (19). Furthermore, there were limitations in the quality of the studies; for example, at least half of the studies utilised retrospective data, incorporating significant bias (19). The SSC group reported that in view of the lack of high-quality randomised control trials (RCTs) in evaluating CDS paediatric sepsis tools, they would not be able to advocate any specific tool at present (2).
Following the findings of the 2020 SSC guidelines, a paediatric sepsis screening tool was developed and validated across 16 EDs in Queensland, Australia (31). The final model, involving 16 criteria, demonstrated a sensitivity of 90% [95% confidence interval (CI): 87–92%] and a specificity of 50% (95% CI: 49–53%) (31). In the UK, a national audit demonstrated that although 92% of emergency care settings have a CDS tool for paediatric sepsis, its mean usage was only 38%, suggesting possible barriers in utilising it in the ED setting (32). Many clinician-based tools require timely and regular monitoring of results and the patient by staff to detect deteriorating patients. This can prove challenging in the context of busy ED environments or short staffing. However, the widespread implementation of electronic information systems has now made it possible for paediatric sepsis CDS tools to be integrated into computerised systems, which can help address this issue. Single-institution studies have demonstrated that an electronic health record (EHR) based screening tool can yield high sensitivity and, when combined with a senior clinical assessment, can significantly improve specificity (2,33). A 2022 scoping review of computerised clinical decision support (CCDS) tools for paediatric sepsis identified only seven publications and six conference abstracts that were relevant, with only four exploring clinical outcomes associated with these tools (25). Again, the quality and heterogeneity of the limited studies affected overall conclusions to be made (25).
To overcome the misuse of the paediatric CDS tool, the utilisation of an in-built triage-based CCDS tool within two emergency care settings was assessed (34). The CCDS tool in isolation demonstrated a sensitivity of 86.7% (95% CI: 77.5–92.6%) and specificity of 87% (95% CI: 86.5–87.5%) in identifying septic patients, which improved when combined with clinical assessment to a sensitivity of 90% (95% CI: 81.4–95.0%) and specificity of 99.4% (95% CI: 99.3–99.5%) (34). Whilst demonstrating the benefits of CCDS, this also emphasised the necessity of clinical judgement in accurately recognising sepsis (34). Overall, more robustly designed randomized controlled trial (RCT) studies evaluating CDS paediatric sepsis tools are required to also evaluate the effects on sepsis care processes and patient outcomes, with better consensus in paediatric sepsis definitions to improve heterogeneity of the data (35). With recent advances in AI, CCDS are likely to increase as vast data sets can be analysed to help guide the detection of subtle signs and patterns of sepsis that could otherwise be overlooked (35,36). However, it is essential to thoroughly evaluate and validate these models, as well as to educate clinicians on their appropriate use, before any real-world implementations (35).
Meanwhile, efforts are underway to develop sepsis CDS tools for resource-poor settings, with their use being essential for improving universal health-care access (37). However, the resources, training, and capabilities of LMICs are heterogeneous between different countries and regionally within the same country (6,11,35). This makes the development of a one-size-fits-all tool challenging. High digital maturity disparities in LMICs create further adoption challenges (35).
Sepsis biomarkers
In the ED, there are several biomarkers which can be considered when discriminating a bacterial aetiology of a fever and, when predicting poor outcomes (Figure 2).
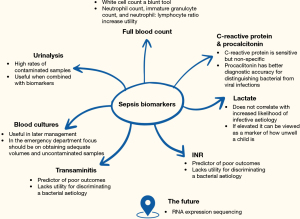
The availability and operationalisation of biomarkers vary significantly depending on the context, resources, setting, and clinician expertise (38). For instance, in HICs and UMICs, early presentations of illness are more commonly observed, and resources are more readily available (38). In contrast, in the low and LMICs, facilities may be remote, presentations occur later, and resources are less accessible (38).
Even within HICs and UMICs, there are significant variations in the use of biomarkers (26,38,39). The specific example of assessing febrile infants under three months of age highlights this variability in practice (26). For instance, in the UK and Ireland, blood and urine testing is advised for all febrile infants (26,40). However, compared to the Paediatric Emergency Care Combined Research Network (PECARN) rule and the American Academy of Pediatrics (AAP) guidelines, procalcitonin (PCT) is rarely available despite both being HICs, somewhat limiting the generalisability of these guidelines to other settings (41-43). There are also knowledge gaps related to phenotype characterisation in different settings. The sepsis phenotype has a wide range of epidemiological differential determinants, including patient-related biological features such as the prevalence of predisposing co-morbidities and disease prevalence, which is also influenced by previous and current therapies, e.g., vaccinations (8,38,44,45).
Full blood count (FBC)
The FBC, which is widely available across healthcare contexts, is a useful tool in the diagnosis of infection in children, but its utility depends on the component part of the FBC used. Perhaps the most basic of these is the total white cell count. It has been demonstrated to have an unadjusted odds ratio (OR) of 6.9 (95% CI: 1.7–28.4) (46). However, even though it has been shown to have good discrimination for infection, an absolute cut-off is difficult due to its non-specific nature (47,48). More information can be gained by looking at the make-up of this total count. For example, the absolute neutrophil count has also proven to be a good tool in discriminating invasive bacterial infection from viral infection (49). However, using this neutrophil count as part of the neutrophil: lymphocyte ratio seems to have better clinical utility (50,51). Use of a neutrophil: lymphocyte ratio of ≥4 may improve the positive likelihood ratio to 12.4 (95% CI: 6.1–25.0) (52). Another feature of haematological analysis which may be useful for the paediatric emergency clinician is the immature granulocyte count. It has been demonstrated to be preferable to total neutrophil count and C-reactive protein (CRP) when appropriate cut-offs have been selected (52) and has been shown to dramatically improve diagnostic yield when used in combination with other biochemical markers (53). It is known that platelet counts may increase during inflammatory processes (54). Increased turnover of platelets during this time may also yield lower overall platelet volumes. Therefore, the platelet: mean platelet volume can be used as a proxy marker of platelet turnover. There is some evidence that this may be especially useful in early presentations, e.g., <12 hours (54). A threshold of 30.0×109/L had a sensitivity of 76.1% when differentiating between bacterial and viral groups if they had arrived at the paediatric ED <12 hours from the onset of fever (54).
Overall, the white cell count should be viewed as a blunt tool, and further discriminatory yield can be had by considering the neutrophil count, immature granulocyte count and neutrophil: lymphocyte ratio. The platelet: mean platelet volume ratio may also be useful in early presentations.
CRP and PCT
CRP has long been used as a biomarker in paediatric sepsis (55). It has demonstrated better sensitivity and specificity than many haematological tests for paediatric sepsis, such as white cell count, absolute neutrophil count, neutrophil: lymphocyte ratio, erythrocyte sedimentation rate and other acute phase reactants (49,50,55,56). It is familiar to clinicians and has good feasibility (57). It is also beneficial due to the correlation between the height of CRP and disease severity (58). However, it is known that CRP has poorer specificity, especially in early disease (59-61). This can create a “clinical lag” and a corresponding biochemical picture behind the presentation. There is also some evidence that it is possible to use CRP to discern viral illness from bacterial illness, although this is limited compared to other biomarkers (49).
PCT, like CRP, has been shown to have better diagnostic accuracy compared to other acute-phase reactants (62). It also seems to be more useful in paediatric populations than in adults (63). However, a 2015 statement by NICE declared that insufficient evidence exists at present regarding the feasibility and cost-efficacy of PCT as a diagnostic marker for general use within healthcare systems (64).
The main benefit of PCT to the paediatric emergency clinician may be in the identification of late-onset neonatal sepsis. There is high-quality evidence of good diagnostic accuracy compared to CRP in populations of less than 3 months of age, and it even has excellent utility as a point-of-care test in this age group (60,61,65,66). However, the discerning clinician must be aware that levels may be elevated within the first 72 hours of life due to physiological reasons surrounding delivery (67).
When considering older children, the evidence is mixed, with studies both finding that PCT had parity with CRP or better diagnostic accuracy than CRP in these age groups (51,57,59,68,69). The evidence broadly agrees that PCT is superior in a shorter history of fever and, given the lack of its upregulation by interferon-γ, a better discriminator of bacterial infection from viraemia (62,69,70). However, care must be taken when interpreting such diagnostic studies, as they have been shown to be often over-enthusiastic when reporting diagnostic accuracy (71). Such studies may also lack validity in the LMIC setting (38).
Overall, both CRP and PCT have limited sensitivity in differentiating bacterial and viral infections. Their use should be in the setting of rigorous clinical assessment and taking into account patient characteristics.
Lactate
Lactate is routinely used as a marker of metabolic stress. Although more readily available than PCT, the international survey involving 2,835 respondents still demonstrated that serum lactate was unavailable for 14% of respondents (11). Lactate has shown good efficacy in predicting the severity of illness, disease course and length of stay (72,73). However, there is recent debate about its efficacy as a prognostic marker in the identification of unwell children (66). Recent evidence suggests that accepted thresholds of 2–2.5 mmol/L may be too low in paediatric populations (74). This is further supported by previous evidence suggesting that a lactate ≥4 had a much higher positive likelihood ratio compared to a threshold of ≥2 at predicting organ failure, requirement for inotropic support, and need for mechanical ventilation (66).
However, the evidence base has demonstrated that lactate is not a good biomarker when specifically identifying paediatric sepsis (66,67,75). It has high specificity in some small numbers of work, but even this supportive work only demonstrated a sensitivity of 33% (67). This is in agreement with the SSC guideline, where lactate was not included due to insufficient evidence of its efficacy as a biomarker in the identification of sepsis (2). If elevated, it should, therefore, be viewed as a marker of how unwell the presenting child is rather than increasing the likelihood of infective aetiology.
International normalised ratio (INR)
Deranged synthetic liver function is a predictor of poor outcomes. An INR ≥1.2 indicates a likelihood of organ dysfunction OR 77 (95% CI: 29–187) (66). This is unsurprising given the mechanistic implications of deranged clotting in the context of the septic patient (76). It is also strongly predictive of the need for inotropic support OR 171 (95% CI: 19–2,063) and the need for mechanical ventilation OR 114 (95% CI: 14–862) (66).
Transaminitis
Transaminitis has demonstrated an increased relative risk of severe disease and has been proposed as a potential early marker (72). It is also used in some validated tools when predicting severe disease (72). However, there is limited evidence to support its use in isolation.
Blood culture
The blood culture, the least useful test to the emergency clinician at the time of index presentation, is often used for targeted antibiotic therapy, including course duration, during the inpatient stay. However, there are significant benefits to having good prior knowledge of local pathogen epidemiology. This will allow clinicians to perform better antimicrobial stewardship while ensuring coverage for potential pathogens. Large multi-centre data should be collected on common pathogenic trends (73).
However, the main consideration for the paediatric emergency clinician at the point of care should be obtaining a good quality sample. Up to 54% of paediatric blood cultures are filled beneath minimally validated volumes, and up to 96.9% are sub-optimally filled (77,78). Multiple, simple, effective strategies are evidenced in improving sample quality (47,48,52,77,78). Under-filled blood culture samples are more likely to produce a contaminant result and less likely to identify a true pathogen (47). This is particularly important as low-level bacteraemia may be more prevalent in paediatric populations whilst remaining clinically relevant (53). The evidence broadly agrees with the following suggested volumes (52,77,79):
- <1 month: ≥0.5 mL;
- 1 month–3 years: ≥1 mL;
- >3 years: ≥4 mL.
Other determinants of adequate blood culture sampling, such as time to incubation, are also likely to be outside the emergency clinician’s control (47). Therefore, care should be taken by the prudent clinician to achieve these volumes.
Urinalysis
The use of the urine dipstick has long been a contentious issue within the paediatric ED, especially given the high rates of contaminant cultures within the infant population (80-82). A commonly held belief is that catheter-obtained samples result in lower contamination rates (80,82). However, a statistically significant difference is not observed in the evidence base (80,82). There is, therefore, debate about whether urinalysis has any role in identifying urinary tract infections or invasive infections in young infants. Urinalysis, when combined with other biomarkers such as PCT and CRP, has demonstrated excellent efficacy in large multi-centre data at differentiating infants at high- and low-risk of invasive disease and, therefore, may be an adjunctive tool to identify inpatients suitable for outpatient management with as many as 1-in-4 infants potentially being able to be treated as outpatients (60).
RNA expression sequencing
Although in the early stages of use in paediatric sepsis, RNA sequencing of expressed biomarkers in inflammatory signalling pathways warrants further study. Currently, feasibility work has been conducted, demonstrating early promise for the technology. This field has the potential to change sepsis diagnostics within the ED (83).
Sepsis phenotypes
Clinical presentations and underlying pathobiology of paediatric sepsis demonstrate high heterogeneity (84). The focus of recent research has been trying to unravel this heterogeneity by identifying subsets of children with phenotypically similar traits (groups of patients with shared clinical features) (84). Characterising sepsis phenotypes has the theoretical potential to prognosticate patients and deliver targeted, more precise sepsis care, including the use of highly specific antimicrobial cover and immune adjuvant therapies, with improved mortality and morbidity outcomes (85).
Current research groups have characterised phenotypes at the genomic, proteomic, immune cell, organ system and clinical-physiological levels, with most studies requiring further validation in larger patient groups to determine prognostic and therapeutic relevance (85,86). Furthermore, these approaches that require genomic or specialised biomarker analyses affect their widespread implementation in all paediatric emergency care settings in view of being too complex, time-consuming and costly (35).
Utilisation of more widespread tools and data to formulate phenotypes, such as routinely collected clinical observations, blood tests, and risk factors, may have a higher impact in more emergency clinical settings, including rural areas and LMICs (35,87).
Sepsis registries
The performance of any screening or diagnostic tool depends on understanding and collating the prevalence, incidence and risk factors of paediatric sepsis within local populations. Currently, no such process exists in the UK, and the NICE has highlighted an urgent need to record accurate epidemiological paediatric sepsis data (3). This could potentially be achieved by developing national sepsis registries, which would allow us to understand the scale of paediatric sepsis faced, provide information to facilitate its early recognition and evaluate and improve emergency care services in their early sepsis management (88).
The Promoting Global Research Excellence in Severe Sepsis (PROGRESS) study developed an international sepsis registry across intensive care units (ICUs) and highlighted the wide variation in outcome and approach to sepsis between countries (89). Thus, the registry is significant in evaluating and improving adherence to current treatment recommendations and identifying and sharing areas of good clinical practice (89). However, despite this 2009 registry’s inclusion of 12,881 patients from 276 ICUs across 37 different countries, there was an overall bias towards HICs, with eight countries accounting for 65.2% of patients and 48.6% of the patients being Caucasian, highlighting healthcare inequity (89). Given that this study took place before the rise of digital technology and in light of shifts in global disease patterns, the relevance of the findings for today’s sepsis care may be limited (8,35,89).
The feasibility and difficulties of developing a sepsis registry for paediatric emergency care settings were explored in the UK’s Piloting a Registry for Paediatric Sepsis (PoRPoise) study (88). After developing a compact minimum viable product (MVP) containing expert consensus-agreed core information pertaining to sepsis in children, it was trialled across three London-based emergency care settings over a 10-week period (88). Several obstacles that affected the collection of critical data emerged at local sites, such as limitations in finding the data, difficulty obtaining scanned data, or unspecific data due to broad administrative coding (88). Broader impediments in setting up a national sepsis registry across the National Health Service (NHS) have been reported in Northern Ireland due to considerable barriers in collating large volumes of clinical data and lack of computerised infrastructure, governance framework, and compliance with the General Data Protection Regulation (GDPR) (90).
Although developing a UK paediatric sepsis registry demonstrates clear benefits, it would require considerable financial provision and collaboration of key stakeholders, including the public and politicians, to overcome hurdles and come to fruition (88,90). This may be a future area of development as the accessibility, efficacy, and reliability of AI continue to improve (35,36).
Management of sepsis
Interventions for paediatric sepsis are shown in Figure 3.
Antibiotics
After prompt recognition of sepsis and appropriate culture acquisition [e.g., blood, urine, cerebrospinal fluid (CSF), depending on the clinical manifestations], clinical guidelines recommend initiation of antibiotics (91). For paediatric patients with septic shock, antibiotics should be initiated within 1-hour to achieve optimal outcomes and reduce mortality (2,91). A big change was introduced in the 2020 SSC for paediatric patients (2). Time of antibiotics administration for children with suspected sepsis (e.g., organ dysfunction but not in septic shock) switched from 1 to 3-hour (2). This allows clinicians to closely monitor and perform diagnostic tests in patients with suspected sepsis and children without risk factors for sepsis without increasing the risk for adverse events from broad-spectrum antibiotics and potential antimicrobial resistance (2,91). However, the panel highlights that antibiotics should be immediately administered if clinical manifestations or early diagnostic tests support clinical infection diagnosis (2). Future studies should investigate the balance between antibiotic initiation time for patients without septic shock and optimisation of the diagnostic strategy.
Initial empiric antibiotics should cover a broad range of potential pathogens based on patient clinical manifestations, presence of risk factors (e.g., age, presence of central catheter, immunocompromised patients, concomitant diseases) and local epidemiology (2,4,92).
De-escalation, narrowing and total duration of antibiotics should be discussed daily in view of cultures’ results and patient clinical course as shown in prior quality improvement studies (93,94).
Fluids
Following the Fluid Expansion As Supportive Therapy (FEAST) trial performed in sub-Saharan Africa, including paediatric patients who presented with sepsis and signs of abnormal perfusion, recommendations regarding fluid bolus were updated (95). Results of the trial showed a significant increase in 48-hour mortality in patients who received fluid bolus versus placebo (95). However, a cautious interpretation of the results due to the limited external validity of findings and the lack of paediatric intensive care unit (PICU) availability should be implemented (95,96). This was also evident from a study that compared paediatric sepsis guidelines, which showed that, regardless of the findings of the FEAST trial, current recommendations suggest the use of fluid bolus at a rate of 10–20 mL/kg in some of the guidelines (97). A pilot study on Fluids in Shock (FiSh) performed in the UK compared fluid bolus of 10 mL/kg (restricted) versus 20 mL/kg (standard therapy) (98). Results showed no differences between groups (98). However, a larger trial could not be performed due to patients’ characteristics and trial design restrictions (98). The SSC provides three main recommendations based on the interplay between clinical manifestations and PICU availability (2). For children with clinical signs of septic shock and availability of intensive care support, 40–60 mL/kg fluid boluses (10–20 mL/kg per bolus) within the first hour may be administered (2). When intensive care is unavailable, children with sepsis without hypotension should not receive a fluid bolus (2). In contrast, a 40 mL/kg bolus (10–20 mL/kg per bolus) should be administered if hypotension is present (2). Regardless of fluid bolus strategy, closed monitoring and rigorous clinical re-assessment for signs of fluid responsiveness [e.g., blood pressure, heart rate, capillary refill time (CRT), urine output] or fluid overload (e.g., presence of crackles, respiratory distress, hepatomegaly radiographic findings of pulmonary oedema) should be implemented (2).
There is an ongoing debate regarding the optimal crystalloid choice as initial resuscitation fluids in septic shock. In recent guidelines and the SSC recommendations, balanced crystalloids (e.g., Ringer’s lactate, Plasma-Lyte) were recommended based on low-quality evidence (2,99). There is a paucity of paediatric RCTs comparing balanced crystalloids to 0.9% normal saline. Of note, two observational cohort studies including 30,532 paediatric patients treated for sepsis showed lower mortality rates in a balanced group compared to the 0.9% normal saline group (OR, 0.79; 95% CI: 0.65–0.95) (2,100,101). There were no differences regarding rates of acute kidney injury between groups (100,101). Adult RCTs in critically ill patients showed a favourable outcome in patients treated with balanced crystalloids compared to 0.9% normal saline regarding the need for renal replacement therapy, major adverse kidney events and mortality (102,103). Currently, the PRagMatic Pediatric Trial of Balanced versus nOrmaL saline fLUid in Sepsis (PRoMPT BOLUS) study is underway, which is an international multicentre RCT comparing balanced versus 0.9% normal saline use in paediatric patients between 6-month-old and <18-year-old presenting in the paediatric ED with septic shock (104). Pending high-quality paediatric data, current recommendations favour using balanced crystalloids over 0.9% normal saline with the exceptions of specific indications (e.g., patients with hyponatremia).
Vasoactive medications
Vasoactive medications are primarily used to increase cardiac output and tissue oxygenation by enhancing the cardiac muscle’s contractile force (2,105). There are different types of vasoactive medications used in septic shock (Figure 4).
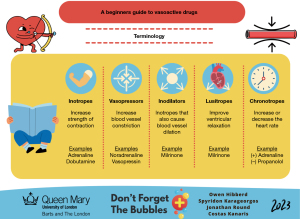
The SSC recommends the use of either adrenaline or noradrenaline as the initial inotrope in children who have already received fluid resuscitation without adequate clinical improvement (2). There is no available evidence comparing outcomes between the two inotropes, and consequently, the initial choice depends on the patient’s pathophysiology, departmental practice and clinicians’ preferences (2). Typically, adrenaline is preferred when the goal is to increase cardiac output in view of myocardial dysfunction, whereas noradrenaline is preferred for increasing systemic vascular resistance (2). However, the availability of vasoactive medications and PICU services is highly variable, with health care inequity existing in LMICs (7,11).
There is a paucity of data regarding the optimal time for initiating inotropes in paediatric septic shock (2). Results of an ongoing paediatric RCT comparing the early initiation of inotropes (fluid-sparing group) versus starting inotropes after 60 mL/kg fluid boluses (liberal fluid group) are expected (106). Current recommendations suggest the use of inotropes after 40–60 mL/kg resuscitation fluids while also highlighting the importance of rigorous clinical reassessment after each fluid bolus for signs of fluid overload (2).
Although the preferred route of inotropes administration should be central [e.g., intraosseous (IO) or central venous line], current guidelines suggest the initial use of vasoactive medications peripherally (with appropriate diluted concentrations) while central access is pending, especially in the paediatric ED or non-PICU settings (2,105).
Adjunctive therapies
Beyond sepsis bundles, including the well-established combination of first-line therapies detailed above for the initial treatment and management, the SSC reviewed the evidence for a variety of advanced therapies for patients with refractory septic shock (2).
Corticosteroids
Intravenous (IV) hydrocortisone could serve as a therapeutic option for septic shock due to the variety of roles cortisol plays in physiological homeostasis. These include increasing noradrenaline reuptake, upregulating cardiac adrenoreceptors, and enhancing vascular and myocardial contractility (107,108). However, corticosteroid therapy is also associated with side effects ranging from hyperglycaemia to an increased incidence of hospital-acquired infections (109,110).
An RCT showed that corticosteroid administration shortened the duration of shock without an increase in mortality or higher incidence of hospital-acquired infection (111). Conversely, an RCT involving 101 patients found no significant difference between duration of inotrope treatment, mechanical ventilation, PICU and hospital length of stay between patients who received corticosteroids versus placebo (112). A large meta-analysis involving 42 RCTs, of which the majority were adult patients, demonstrated that the use of corticosteroid led to a small reduction in mortality, faster resolution of shock and a shorter length of stay (113). They did, however, note an increased risk of corticosteroid-related side effects (113).
Overall, due to a lack of high-quality, large-scale studies clearly demonstrating benefit with corticosteroid treatment, there is insufficient evidence to support the use of corticosteroids for sepsis treatment and the SSC guidelines recommend against the use of IV hydrocortisone in the treatment of septic shock responsive to fluids and inotropes, in the absence of suspected or proven adrenal insufficiency (2). However, hydrocortisone may be considered if inotropes and fluids are insufficient in reversing haemodynamic instability (2).
Insulin and glucose
Hyperglycaemia correlates with poor outcomes in critically unwell patients. Numerous studies investigated whether glycaemic control via administration of IV insulin could therefore be of benefit in the paediatric population (2). Several large RCTs with relatively low glucose targets failed to show improvement in outcomes in PICU patients (114,115). This is also supported by meta-analyses that also demonstrated a higher incidence of hypoglycaemia when aiming for glucose levels below 7.8 mmol/L (140 mg/dL) (116,117).
The SSC guidelines strongly recommend against insulin administration due to the risk of hypoglycaemia when aiming for a glucose of 7.8 mmol/L or less (2,114,115). The level of monitoring required to utilise this therapy safely may also reduce its utility in an LMIC setting (11,115). Guidelines do, however, suggest potential use when an upper glucose target of 10 mmol/L (180 mg/dL) is used (2,114,115).
Calcium
Calcium plays a pivotal role in multiple processes implicated in septic shock, including myocardial contractility and the maintenance of vasomotor tone (118,119). A prospective cohort study monitoring the prevalence and implications of hypocalcaemia in PICU patients showed that over three-quarters of patients had low calcium levels (120). Hypocalcaemia in this study was associated with higher severity of organ dysfunction, although no association with mortality was observed (120). There are currently no studies investigating the potential benefit of calcium supplementation in paediatric patients with septic shock, and as such, no recommendation regarding the use of calcium in septic shock has been made (2).
Blood products
Optimal haemoglobin levels required for adequate tissue oxygen delivery were proposed as a potential component of managing the septic child (2). A post hoc subgroup analysis study of paediatric patients with sepsis was performed using data from the Transfusion Requirements in the PICU (TRIPICU) study (121). In this analysis, hemodynamically stable PICU patients with sepsis were randomised to receive blood transfusions when their haemoglobin levels fell to either 7.0 g/dL (the restrictive group) or 9.5 g/dL (the liberal group) (121). There was no difference found in rates of new or progression of ongoing multiple organ dysfunction, and there was no statistical difference in mortality rates between the two groups (121). As such, the SSC guidelines suggest that hemodynamically stable septic patients should be transfused only if haemoglobin levels fall below 7.0 g/dL (2). Moreover, due to the lack of evidence identifying a threshold at which the benefits of platelet transfusions to prevent spontaneous bleeds in sepsis-induced disseminated intravascular coagulation (DIC) outweigh the risks, the SSC suggests against routine prophylactic transfusion (2). Finally, there is also a lack of data regarding the benefits of prophylactic plasma transfusions, and as such, the recommendation is against this practice (2).
Conclusions
Significant advances in paediatric sepsis recognition and management are expected to integrate sepsis phenotypes and biomarker profiles into protocols consistently. Registries could enhance our population-level understanding of sepsis and address healthcare disparities. Future research should prioritise high-quality RCTs, with digital health and AI showing particular promise.
Acknowledgments
The authors would like to acknowledge the “Don’t Forget The Bubbles” team, in particular Andy Tagg, Becky Platt, Dani Hall, and Tessa Davis for their ongoing support of collaborative paediatric emergency medicine research and reviews.
Footnote
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-24-70/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-24-70/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Minogue J, Keogh S, Schlapbach LJ, et al. Long-term outcomes after paediatric sepsis: A narrative review. Aust Crit Care 2024;37:499-507. [Crossref] [PubMed]
- Weiss SL, Peters MJ, Alhazzani W, et al. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr Crit Care Med 2020;21:e52-e106. [Crossref] [PubMed]
- National Institute of Health and Care Excellence. Suspected sepsis: recognition, diagnosis and early management. 2016. Available online: https://www.nice.org.uk/guidance/ng51
- Miranda M, Nadel S. Pediatric Sepsis: a Summary of Current Definitions and Management Recommendations. Curr Pediatr Rep 2023;11:29-39. [Crossref] [PubMed]
- Schlapbach LJ, Watson RS, Sorce LR, et al. International Consensus Criteria for Pediatric Sepsis and Septic Shock. JAMA 2024;331:665-74. [Crossref] [PubMed]
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 2020;395:200-11. [Crossref] [PubMed]
- Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, et al. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med 2018;6:223-30. [Crossref] [PubMed]
- Tan B, Wong JJ, Sultana R, et al. Global Case-Fatality Rates in Pediatric Severe Sepsis and Septic Shock: A Systematic Review and Meta-analysis. JAMA Pediatr 2019;173:352-62. [Crossref] [PubMed]
- Menon K, Schlapbach LJ, Akech S, et al. Criteria for Pediatric Sepsis—A Systematic Review and Meta-Analysis by the Pediatric Sepsis Definition Taskforce. Crit Care Med 2022;50:21-36. [Crossref] [PubMed]
- Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6:2-8. [Crossref] [PubMed]
- Morin L, Hall M, de Souza D, et al. The Current and Future State of Pediatric Sepsis Definitions: An International Survey. Pediatrics 2022;149:e2021052565. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Sanchez-Pinto LN, Bennett TD, DeWitt PE, et al. Development and Validation of the Phoenix Criteria for Pediatric Sepsis and Septic Shock. JAMA 2024;331:675-86. [Crossref] [PubMed]
- Lanziotti VS, Ventura A, Kache S, et al. New Phoenix criteria for pediatric sepsis and septic shock: the strengths and the future of a comprehensive perspective. Crit Care Sci 2024;36:e20240058en.
- Harley A, Schlapbach LJ, Johnston ANB, et al. Challenges in the recognition and management of paediatric sepsis - The journey. Australas Emerg Care 2022;25:23-9. [Crossref] [PubMed]
- Roland D, Munro A. Time for paediatrics to screen out sepsis “screening”. BMJ 2023;381:1327. [Crossref] [PubMed]
- Nijman RG, Vergouwe Y, Thompson M, et al. Clinical prediction model to aid emergency doctors managing febrile children at risk of serious bacterial infections: diagnostic study. BMJ 2013;346:f1706. [Crossref] [PubMed]
- Martin AJ, van der Velden FJS, von Both U, et al. External validation of a multivariable prediction model for identification of pneumonia and other serious bacterial infections in febrile immunocompromised children. Arch Dis Child 2023;109:58-66. [Crossref] [PubMed]
- Oruganti S, Evans J, Cromarty T, et al. Identification of sepsis in paediatric emergency departments: A scoping review. Acta Paediatr 2022;111:2262-77. [Crossref] [PubMed]
- Brennan L, Heal C, Brown S, et al. Time to change the reference ranges of children’s physiological observations in emergency care? A prospective study. J Paediatr Child Health 2023;59:480-6. [Crossref] [PubMed]
- Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 2011;377:1011-8. [Crossref] [PubMed]
- O’Leary F, Hayen A, Lockie F, et al. Defining normal ranges and centiles for heart and respiratory rates in infants and children: a cross-sectional study of patients attending an Australian tertiary hospital paediatric emergency department. Arch Dis Child 2015;100:733-7. [Crossref] [PubMed]
- Bonafide CP, Brady PW, Keren R, et al. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics 2013;131:e1150-7. [Crossref] [PubMed]
- Theodosiou AA, Mashumba F, Flatt A. Excluding Clinically Significant Bacteremia by 24 Hours in Otherwise Well Febrile Children Younger Than 16 Years: A Study of More Than 50,000 Blood Cultures. Pediatr Infect Dis J 2019;38:e203-8. [Crossref] [PubMed]
- Ackermann K, Baker J, Green M, et al. Computerized Clinical Decision Support Systems for the Early Detection of Sepsis Among Adult Inpatients: Scoping Review. J Med Internet Res 2022;24:e31083. [Crossref] [PubMed]
- Wilcox H, Umana E, Fauteux-Lamarre E, et al. Conundrums in the Management of Febrile Infants under Three Months of Age and Future Research. Antibiotics (Basel) 2024;13:88. [Crossref] [PubMed]
- Powell R, Jeavons K. Identifying paediatric sepsis: the difficulties in following recommended practice and the creation of our own pathway. Arch Dis Child 2018;103:114. [Crossref] [PubMed]
- Carter MJ, Stilwell PA, Nijman RG, et al. Identification and treatment of paediatric sepsis: getting the balance right. Arch Dis Child 2018;103:1185-6. [Crossref] [PubMed]
- Roland D, Stohr W, Gibb D, et al. Evidence of Poor Utility of Current Sepsis Screening Tools in an At-Risk Population of Children With Community-Acquired Pneumonia. Pediatr Infect Dis J 2025; Epub ahead of print. [Crossref] [PubMed]
- Bielicki JA, Stöhr W, Barratt S, et al. Effect of Amoxicillin Dose and Treatment Duration on the Need for Antibiotic Re-treatment in Children With Community-Acquired Pneumonia: The CAP-IT Randomized Clinical Trial. JAMA 2021;326:1713-24. [Crossref] [PubMed]
- Gilholm P, Gibbons K, Lister P, et al. Validation of a paediatric sepsis screening tool to identify children with sepsis in the emergency department: a statewide prospective cohort study in Queensland, Australia. BMJ Open 2023;13:e061431. [Crossref] [PubMed]
- The Royal College of Emergency Medicine. Feverish Child—National Quality Improvement Project. Available online: https://rcem.ac.uk/wp-content/uploads/2021/10/RCEM_Feverish_Child_National_Report_July2019.pdf
- Balamuth F, Alpern ER, Grundmeier RW, et al. Comparison of Two Sepsis Recognition Methods in a Pediatric Emergency Department. Acad Emerg Med 2015;22:1298-306. [Crossref] [PubMed]
- Gomes S, Wood D, Ayis S, et al. Evaluation of a novel approach to recognising community-acquired paediatric sepsis at ED triage by combining an electronic screening algorithm with clinician assessment. Emerg Med J 2021;38:132-8. [Crossref] [PubMed]
- Sanchez-Pinto LN, Del Pilar Arias López M, Scott H, et al. Digital solutions in paediatric sepsis: current state, challenges, and opportunities to improve care around the world. Lancet Digit Health 2024;6:e651-61. [Crossref] [PubMed]
- Tennant R, Graham J, Kern J, et al. A scoping review on pediatric sepsis prediction technologies in healthcare. NPJ Digit Med 2024;7:353. [Crossref] [PubMed]
- Jimenez-Zambrano A, Ritger C, Rebull M, et al. Clinical decision support tools for paediatric sepsis in resource-poor settings: an international qualitative study. BMJ Open 2023;13:e074458. [Crossref] [PubMed]
- Carrol ED, Ranjit S, Menon K, et al. Operationalizing Appropriate Sepsis Definitions in Children Worldwide: Considerations for the Pediatric Sepsis Definition Taskforce. Pediatr Crit Care Med 2023;24:e263-71. [Crossref] [PubMed]
- Umana E, Mills C, Norman-Bruce H, et al. Performance of clinical decision aids (CDA) for the care of young febrile infants: a multicentre prospective cohort study conducted in the UK and Ireland. EClinicalMedicine 2024;78:102961. [Crossref] [PubMed]
- National Institute for Health and Care Excellence. Urinary tract infection in under 16s: diagnosis and management. 2022. Available online: https://www.nice.org.uk/guidance/ng224
- Umana E, Norman-Bruce H, Mills C, et al. Applying the American Academy of Pediatrics guideline to a cohort of febrile infants attending emergency departments in the UK and Ireland. Eur J Emerg Med 2023;30:219-21. [Crossref] [PubMed]
- Pantell RH, Roberts KB, Adams WG, et al. Evaluation and Management of Well-Appearing Febrile Infants 8 to 60 Days Old. Pediatrics 2021;148:e2021052228. [Crossref] [PubMed]
- Kuppermann N, Dayan PS, Levine DA, et al. A Clinical Prediction Rule to Identify Febrile Infants 60 Days and Younger at Low Risk for Serious Bacterial Infections. JAMA Pediatr 2019;173:342-51. [Crossref] [PubMed]
- Battersby AJ, Knox-Macaulay HH, Carrol ED. Susceptibility to invasive bacterial infections in children with sickle cell disease. Pediatr Blood Cancer 2010;55:401-6. [Crossref] [PubMed]
- Menon K, Sorce LR, Argent A, et al. Reporting of Social Determinants of Health in Pediatric Sepsis Studies. Pediatr Crit Care Med 2023;24:301-10. [Crossref] [PubMed]
- Goh PL, Lee SW, Wong EH. Predictors of serious bacterial infection in children aged 3 to 36 months with fever without source. Singapore Med J 2006;47:276-80. [PubMed]
- Whelan SO, Mulrooney C, Moriarty F, et al. Pediatric blood cultures—turning up the volume: a before and after intervention study. Eur J Pediatr 2024;183:3063-71. [Crossref] [PubMed]
- Ohnishi T, Kamimaki I, Kobayashi R, et al. Verification of blood volume for blood culture and detection rate in pediatrics. J Infect Chemother 2020;26:471-4. [Crossref] [PubMed]
- Bal A, Anil M, Gökalp G, B, et al. Comparison of the Eosinophil Count to C –reactive protein, Leukocyte Count, and Neutrophil Count for the detection of bacterial infection in ill-appearing children with fever admitted to the Emergency Department. Signa Vitae 2015;10:163-76. [Crossref]
- Chang SSY, Lim AZ, Ong GY, et al. Predictors of serious bacterial infections using serum biomarkers in an infant population aged 0 to 90 days: a prospective cohort study. BMJ Paediatr Open 2021;5:e000861. [Crossref] [PubMed]
- Gunduz A, Tekin M, Konca C, et al. Effectiveness of Laboratory Markers in Determining Serious Bacterial Infection in Children with Fever without Source. J Pediatr Infect Dis 2018;13:287-92. [Crossref]
- Allen E, Cavallaro A, Keir AK. A Quality Improvement Initiative to Reduce Blood Culture Contamination in the Neonatal Unit. Pediatr Qual Saf 2021;6:e413. [Crossref] [PubMed]
- Kellogg JA, Manzella JP, Bankert DA. Frequency of low-level bacteremia in children from birth to fifteen years of age. J Clin Microbiol 2000;38:2181-5. [Crossref] [PubMed]
- Tamelytė E, Vaičekauskienė G, Dagys A, et al. Early Blood Biomarkers to Improve Sepsis/Bacteremia Diagnostics in Pediatric Emergency Settings. Medicina (Kaunas) 2019;55:99. [Crossref] [PubMed]
- Pulliam PN, Attia MW, Cronan KM. C-reactive protein in febrile children 1 to 36 months of age with clinically undetectable serious bacterial infection. Pediatrics 2001;108:1275-9. [Crossref] [PubMed]
- dos Anjos BL, Grotto HZ. Evaluation of C-reactive protein and serum amyloid A in the detection of inflammatory and infectious diseases in children. Clin Chem Lab Med 2010;48:493-9. [Crossref] [PubMed]
- Andreola B, Bressan S, Callegaro S, et al. Procalcitonin and C-reactive protein as diagnostic markers of severe bacterial infections in febrile infants and children in the emergency department. Pediatr Infect Dis J 2007;26:672-7. [Crossref] [PubMed]
- Gendrel D, Raymond J, Coste J, et al. Comparison of procalcitonin with C-reactive protein, interleukin 6 and interferon-alpha for differentiation of bacterial vs. viral infections. Pediatr Infect Dis J 1999;18:875-81. [Crossref] [PubMed]
- Luaces-Cubells C, Mintegi S, García-García JJ, et al. Procalcitonin to detect invasive bacterial infection in non-toxic-appearing infants with fever without apparent source in the emergency department. Pediatr Infect Dis J 2012;31:645-7. [Crossref] [PubMed]
- Velasco R, Gomez B, Labiano I, et al. Performance of Febrile Infant Algorithms by Duration of Fever. Pediatrics 2024;153:e2023064342. [Crossref] [PubMed]
- Olaciregui I, Hernández U, Muñoz JA, et al. Markers that predict serious bacterial infection in infants under 3 months of age presenting with fever of unknown origin. Arch Dis Child 2009;94:501-5. [Crossref] [PubMed]
- Pourakbari B, Mamishi S, Zafari J, et al. Evaluation of procalcitonin and neopterin level in serum of patients with acute bacterial infection. Braz J Infect Dis 2010;14:252-5. [Crossref] [PubMed]
- Mills GD, Lala HM, Oehley MR, et al. Elevated procalcitonin as a diagnostic marker in meningococcal disease. Eur J Clin Microbiol Infect Dis 2006;25:501-9. [Crossref] [PubMed]
- National Institute for Health and Care Excellence. Procalcitonin testing for diagnosing and monitoring sepsis (ADVIA Centaur BRAHMS PCT assay, BRAHMS PCT Sensitive Kryptor assay, Elecsys BRAHMS PCT assay, LIAISON BRAHMS PCT assay and VIDAS BRAHMS PCT assay). 2015. Available online: https://www.nice.org.uk/guidance/dg18
- Norman-Bruce H, Umana E, Mills C, et al. Diagnostic test accuracy of procalcitonin and C-reactive protein for predicting invasive and serious bacterial infections in young febrile infants: a systematic review and meta-analysis. Lancet Child Adolesc Health 2024;8:358-68. [Crossref] [PubMed]
- Long E, Solan T, Stephens DJ, et al. Febrile children in the Emergency Department: Frequency and predictors of poor outcome. Acta Paediatr 2021;110:1046-55. [Crossref] [PubMed]
- Elie-Turenne M, Sahari I, Baricella R, et al. Lactate as a Predictor of Admission In Emergency Department Pediatric Sepsis. Ann Emerg Med 2010;56:S47-8. [Crossref]
- Waterfield T, Maney JA, Lyttle MD, et al. Diagnostic test accuracy of point-of-care procalcitonin to diagnose serious bacterial infections in children. BMC Pediatr 2020;20:487. [Crossref] [PubMed]
- Moldovan DA, Baghiu MD, Balas A, et al. The Value of the “Lab-Score” Method in Identifying Febrile Infants at Risk for Serious Bacterial Infections. J Crit Care Med (Targu Mures) 2015;1:11-7. [Crossref] [PubMed]
- Mantadakis E, Plessa E, Vouloumanou EK, et al. Serum procalcitonin for prediction of renal parenchymal involvement in children with urinary tract infections: a meta-analysis of prospective clinical studies. J Pediatr 2009;155:875-881.e1. [Crossref] [PubMed]
- Reed L, Carroll J, Cummings A, et al. Serum lactate as a screening tool and predictor of outcome in pediatric patients presenting to the emergency department with suspected infection. Pediatr Emerg Care 2013;29:787-91. [Crossref] [PubMed]
- Sepanski RJ, Godambe SA, Mangum CD, et al. Designing a pediatric severe sepsis screening tool. Front Pediatr 2014;2:56. [Crossref] [PubMed]
- Gomez B, Hernandez-Bou S, Garcia-Garcia JJ, et al. Bacteremia in previously healthy children in emergency departments: clinical and microbiological characteristics and outcome. Eur J Clin Microbiol Infect Dis 2015;34:453-60. [Crossref] [PubMed]
- Nygaard U, Dungu KHS, von Linstow ML, et al. Lactate as a Screening Tool for Critical Illness in a Pediatric Emergency Department. Pediatr Emerg Care 2023;39:735-8. [Crossref] [PubMed]
- Scott HF, Donoghue AJ, Gaieski DF, et al. The utility of early lactate testing in undifferentiated pediatric systemic inflammatory response syndrome. Acad Emerg Med 2012;19:1276-80. [Crossref] [PubMed]
- Semeraro N, Ammollo CT, Semeraro F, et al. Coagulopathy of Acute Sepsis. Semin Thromb Hemost 2015;41:650-8. [Crossref] [PubMed]
- Harewood FC, Curtis N, Daley AJ, et al. Adequate or Inadequate? The Volume of Blood Submitted for Blood Culture at a Tertiary Children’s Hospital. Clin Pediatr (Phila) 2018;57:1310-7. [Crossref] [PubMed]
- Singh MP, Balegar V KK, Angiti RR. The practice of blood volume submitted for culture in a neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed 2020;105:600-4. [Crossref] [PubMed]
- Connell TG, Rele M, Cowley D, et al. How reliable is a negative blood culture result? Volume of blood submitted for culture in routine practice in a children’s hospital. Pediatrics 2007;119:891-6. [Crossref] [PubMed]
- Labrosse M, Levy A, Autmizguine J, et al. Evaluation of a New Strategy for Clean-Catch Urine in Infants. Pediatrics 2016;138:e20160573. [Crossref] [PubMed]
- Herreros ML, Gili P, Del Valle R, et al. Urine collection methods for infants under 3 months of age in clinical practice. Pediatr Nephrol 2021;36:3899-904. [Crossref] [PubMed]
- Herr SM, Wald ER, Pitetti RD, et al. Enhanced urinalysis improves identification of febrile infants ages 60 days and younger at low risk for serious bacterial illness. Pediatrics 2001;108:866-71. [Crossref] [PubMed]
- Balamuth F, Alpern ER, Kan M, et al. Gene Expression Profiles in Children With Suspected Sepsis. Ann Emerg Med 2020;75:744-54. [Crossref] [PubMed]
- Papathanakos G, Andrianopoulos I, Xenikakis M, et al. Clinical Sepsis Phenotypes in Critically Ill Patients. Microorganisms 2023;11:2165. [Crossref] [PubMed]
- Seymour CW, Kennedy JN, Wang S, et al. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA 2019;321:2003-17. [Crossref] [PubMed]
- Atreya MR, Huang M, Moore AR, et al. Identification and transcriptomic assessment of latent profile pediatric septic shock phenotypes. Crit Care 2024;28:246. [Crossref] [PubMed]
- Sanchez-Pinto LN, Stroup EK, Pendergrast T, et al. Derivation and Validation of Novel Phenotypes of Multiple Organ Dysfunction Syndrome in Critically Ill Children. JAMA Netw Open 2020;3:e209271. [Crossref] [PubMed]
- Dougherty H, Eisen S, Fraser LK, et al. Piloting a registry for paediatric sepsis: The PoRPoiSe study. J Paediatr Child Health 2022;58:978-84. [Crossref] [PubMed]
- Beale R, Reinhart K, Brunkhorst FM, et al. Promoting Global Research Excellence in Severe Sepsis (PROGRESS): lessons from an international sepsis registry. Infection 2009;37:222-32. [Crossref] [PubMed]
- McCleary D. 680 Developing a paediatric sepsis registry in Northern Ireland. Archives of Disease in Childhood 2022;107:A448-9.
- Karageorgos S, Hibberd O, Mullally PJW, et al. Antibiotic Use for Common Infections in Pediatric Emergency Departments: A Narrative Review. Antibiotics (Basel) 2023;12:1092. [Crossref] [PubMed]
- Mau LB, Bain V. Antimicrobial Therapy in Pediatric Sepsis: What Is the Best Strategy? Front Pediatr 2022;10:830276. [Crossref] [PubMed]
- Weiss CH, Persell SD, Wunderink RG, et al. Empiric antibiotic, mechanical ventilation, and central venous catheter duration as potential factors mediating the effect of a checklist prompting intervention on mortality: an exploratory analysis. BMC Health Serv Res 2012;12:198. [Crossref] [PubMed]
- Weiss CH, Moazed F, McEvoy CA, et al. Prompting physicians to address a daily checklist and process of care and clinical outcomes: a single-site study. Am J Respir Crit Care Med 2011;184:680-6. [Crossref] [PubMed]
- Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011;364:2483-95. [Crossref] [PubMed]
- Duke T. What the African fluid-bolus trial means. Lancet 2011;378:1685-7. [Crossref] [PubMed]
- Dewez JE, Nijman RG, Yeung S. Fluids in the management of sepsis in children: a review of guidelines in the aftermath of the FEAST trial. Arch Dis Child 2019;104:1236. [Crossref] [PubMed]
- Inwald DP, Canter R, Woolfall K, et al. Restricted fluid bolus volume in early septic shock: results of the Fluids in Shock pilot trial. Arch Dis Child 2019;104:426-31. [Crossref] [PubMed]
- Van de Voorde P, Turner NM, Djakow J, et al. European Resuscitation Council Guidelines 2021 Paediatric Life Support. Resuscitation 2021;161:327-87. [PubMed]
- Emrath ET, Fortenberry JD, Travers C, et al. Resuscitation With Balanced Fluids Is Associated With Improved Survival in Pediatric Severe Sepsis. Crit Care Med 2017;45:1177-83. [Crossref] [PubMed]
- Weiss SL, Keele L, Balamuth F, et al. Crystalloid Fluid Choice and Clinical Outcomes in Pediatric Sepsis: A Matched Retrospective Cohort Study. J Pediatr 2017;182:304-310.e10. [Crossref] [PubMed]
- Semler MW, Self WH, Wanderer JP, et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med 2018;378:829-39. [Crossref] [PubMed]
- Self WH, Semler MW, Wanderer JP, et al. Balanced Crystalloids versus Saline in Noncritically Ill Adults. N Engl J Med 2018;378:819-28. [Crossref] [PubMed]
- Weiss SL, Balamuth F, Long E, et al. PRagMatic Pediatric Trial of Balanced vs nOrmaL Saline FlUid in Sepsis: study protocol for the PRoMPT BOLUS randomized interventional trial. Trials 2021;22:776. [Crossref] [PubMed]
- Kanaris C, Wahida R. Inotrope use in children with septic shock: a guide for general paediatricians. Arch Dis Child Educ Pract Ed 2024;109:38-46. [Crossref] [PubMed]
- Parker MJ, Thabane L, Fox-Robichaud A, et al. A trial to determine whether septic shock-reversal is quicker in pediatric patients randomized to an early goal-directed fluid-sparing strategy versus usual care (SQUEEZE): study protocol for a pilot randomized controlled trial. Trials 2016;17:556. [Crossref] [PubMed]
- Seri I, Evans J. Controversies in the diagnosis and management of hypotension in the newborn infant. Curr Opin Pediatr 2001;13:116-23. [Crossref] [PubMed]
- Wehling M. Specific, nongenomic actions of steroid hormones. Annu Rev Physiol 1997;59:365-93. [Crossref] [PubMed]
- Costello JM, Graham DA, Morrow DF, et al. Risk factors for central line-associated bloodstream infection in a pediatric cardiac intensive care unit. Pediatr Crit Care Med 2009;10:453-9. [Crossref] [PubMed]
- Yung M, Wilkins B, Norton L, et al. Glucose control, organ failure, and mortality in pediatric intensive care. Pediatr Crit Care Med 2008;9:147-52. [Crossref] [PubMed]
- El-Nawawy A, Khater D, Omar H, et al. Evaluation of Early Corticosteroid Therapy in Management of Pediatric Septic Shock in Pediatric Intensive Care Patients: A Randomized Clinical Study. Pediatr Infect Dis J 2017;36:155-9. [Crossref] [PubMed]
- Menon K, McNally D, O’Hearn K, et al. A Randomized Controlled Trial of Corticosteroids in Pediatric Septic Shock: A Pilot Feasibility Study. Pediatr Crit Care Med 2017;18:505-12. [Crossref] [PubMed]
- Rochwerg B, Oczkowski SJ, Siemieniuk RAC, et al. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit Care Med 2018;46:1411-20. [Crossref] [PubMed]
- Macrae D, Grieve R, Allen E, et al. A randomized trial of hyperglycemic control in pediatric intensive care. N Engl J Med 2014;370:107-18. [Crossref] [PubMed]
- Agus MS, Wypij D, Hirshberg EL, et al. Tight Glycemic Control in Critically Ill Children. N Engl J Med 2017;376:729-41. [Crossref] [PubMed]
- Zhao Y, Wu Y, Xiang B. Tight glycemic control in critically ill pediatric patients: a meta-analysis and systematic review of randomized controlled trials. Pediatr Res 2018;83:930-5. [Crossref] [PubMed]
- Chen L, Li T, Fang F, et al. Tight glycemic control in critically ill pediatric patients: a systematic review and meta-analysis. Crit Care 2018;22:57. [Crossref] [PubMed]
- Yan D, Xie X, Fu X, et al. U-SHAPED ASSOCIATION BETWEEN SERUM CALCIUM LEVELS AND 28-DAY MORTALITY IN PATIENTS WITH SEPSIS: A RETROSPECTIVE ANALYSIS OF THE MIMIC-III DATABASE. Shock 2023;60:525-33. [Crossref] [PubMed]
- Hibberd O, Price J, Thomas SH, et al. The incidence of admission ionised hypocalcaemia in paediatric major trauma—A systematic review and meta-analysis. PLoS One 2024;19:e0303109. [Crossref] [PubMed]
- Dias CR, Leite HP, Nogueira PC, et al. Ionized hypocalcemia is an early event and is associated with organ dysfunction in children admitted to the intensive care unit. J Crit Care 2013;28:810-5. [Crossref] [PubMed]
- Karam O, Tucci M, Ducruet T, et al. Red blood cell transfusion thresholds in pediatric patients with sepsis. Pediatr Crit Care Med 2011;12:512-8. [Crossref] [PubMed]
Cite this article as: Hibberd O, Karageorgos S, Ranaweera M, Mullally PJW, Athanasiou D, Roland D; the “Don’t Forget The Bubbles” team. Recognising and managing sepsis in children’s emergency care: a clinical practice review. Pediatr Med 2025;8:11.



