
Protective effect of triptolide on puromycin-induced podocyte injury
Highlight box
Key findings
• Triptolide (TPL) exerts a protective effect against puromycin aminonucleoside (PAN)-induced podocyte injury by upregulating the expression of slit diaphragm proteins (nephrin, podocin, and CD2AP) and stabilizing mitochondrial structure and function.
What is known and what is new?
• Podocyte injury is a central pathological feature in various glomerular diseases, and slit diaphragm proteins play crucial roles in maintaining podocyte integrity. Tripterygium wilfordii and its active component TPL have shown therapeutic potential in multiple renal diseases, but the specific mechanisms underlying their protective effects on podocyte injury remain unclear.
• This study demonstrates that TPL can mitigate PAN-induced podocyte injury by stabilizing key slit diaphragm proteins and mitochondrial function, revealing a potential molecular mechanism underlying TPL’s protective role in podocyte damage.
What is the implication, and what should change now?
• The protective effects of TPL provide new therapeutic targets and ideas for podocyte-related kidney diseases. Its mechanism of action through regulating key podocyte proteins and mitochondrial function may offer a theoretical basis for developing novel kidney-protective drugs.
Introduction
Pediatric nephrotic syndrome (NS) is a common renal disorder characterized by massive proteinuria, hypoalbuminemia, hyperlipidemia, and edema. Podocyte injury is a central pathological mechanism underlying NS, and its impact is particularly significant in pediatric populations, where NS is a leading cause of chronic kidney disease (1). In pediatric nephropathy, podocyte effacement and damage are often observed, leading to the disruption of the glomerular filtration barrier and subsequent proteinuria (2,3). Therefore, understanding the mechanisms of podocyte injury and identifying effective therapeutic strategies are crucial for improving outcomes in pediatric patients with NS. Recent study has shown that Tripterygium wilfordii and its active component, triptolide (TPL), exhibit promising therapeutic effects in various renal diseases, including Pediatric NS (4). These agents have been demonstrated to reduce proteinuria by protecting and repairing the glomerular filtration barrier, which is often compromised in NS. Given the central role of podocyte injury in pediatric NS, we hypothesize that TPL may also be beneficial in pediatric nephropathy by targeting podocyte damage and improving renal function.
TPL is an active ingredient extracted from the Chinese herb Tripterygium wilfordii, known for its significant anti-inflammatory, immunosuppressive, and anti-fibrotic effects (4-6). Although TPL has demonstrated certain therapeutic effects in the treatment of adult kidney diseases, its application in pediatric kidney diseases has been relatively less studied. The use of TPL in pediatric refractory NS has gradually attracted attention. Studies have shown that TPL, in combination with glucocorticoids or other immunosuppressive agents, can significantly improve treatment outcomes, reduce proteinuria, and improve renal function (4). Research has found that TPL can significantly lower proteinuria levels, improve renal function, and has relatively good safety (7). However, the toxic and side effects of TPL still need to be carefully assessed. The application of TPL in other pediatric kidney diseases (such as lupus nephritis, Alport syndrome, etc.) is still in the exploratory stage. Current studies indicate that TPL has potential therapeutic value. Despite showing certain therapeutic effects in pediatric kidney diseases, the pathophysiological mechanisms of pediatric kidney diseases differ from those in adults, and children’s tolerance and response to drugs also vary. Therefore, data on the long-term efficacy and safety of TPL in pediatric kidney diseases are limited and further research is still needed. In this study, we aimed to further delineate the molecular biological mechanisms by which TPL protects against glomerular podocyte injury. This was achieved by examining the expression of nephrin, podocin, and CD2AP in podocytes following TPL intervention in a puromycin-induced model, as well as by assessing changes in podocyte morphology and mitochondrial ultrastructure. We present this article in accordance with the MDAR reporting checklist (available at https://pm.amegroups.com/article/view/10.21037/pm-24-73/rc).
Methods
Podocyte culture
The conditionally immortalized mouse podocyte cell line (MPC5) was constructed by Professor Peter Mundel from the United States. The MPC5 cells were cultured following our previously established method (8). The process began with the slow thawing of the podocytes in a warm water bath, extracted from liquid nitrogen storage. Post-thawing, the cells were centrifuged to remove the supernatant within the centrifuge tube. Subsequently, a double antibody solution containing penicillin and streptomycin was added to the cell culture medium to prevent contamination. The thawed podocytes were then transferred to a culture flask pre-filled with this antibiotic-supplemented medium, initiating the proliferation phase of glomerular podocyte culture. The initial culture was conducted at 33 ℃ with 5% CO2 to promote cell growth. This temperature was selected to mimic the physiological conditions that support podocyte proliferation. Once the cells reached a state of active proliferation, the culture temperature was elevated to 37 ℃ to further induce cell differentiation and maturation. This temperature shift is critical for advancing the cells from a proliferative to a differentiated state, which is essential for studying their mature functions and responses. After 14 days of differentiation, the cells were treated with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) (Gibco, Grand Island, NY, USA) to detach them from the culture flask, facilitating the subsequent intervention experiment. The experiment was initiated when the cells achieved approximately 80% confluence, ensuring a sufficient density for meaningful experimental outcomes.
Experimental grouping
The glomerular podocytes were categorized into three distinct groups for the study: the normal control group, the puromycin aminonucleoside (PAN)-induced podocyte injury group (PAN group), and the TPL group. Control group: podocytes in this group were cultured with RPMI 1640 cell culture medium without any additional treatments, serving as a baseline for comparison. PAN group: in this group, the podocytes were exposed to a puromycin-containing medium at a final concentration of 50 µg/mL. Puromycin was sourced from Sigma (St.Louis, MO, USA), and was added to the cell culture flask to induce podocyte injury. TPL group: this group involved a combination treatment, where podocytes were exposed to puromycin at a final concentration of 50 µg/mL along with TPL at a final concentration of 3 ng/mL. The specific treatment condition was denoted as PAN 50 µg/mL + TPL 3 ng/mL. The selection of TPL concentration in this study is based on previous research (9).
Immunofluorescence detection
Following a 24-hour drug treatment, podocytes were washed with pre-cooled phosphate-buffered saline (PBS) to remove any residual drug. Podocytes were fixed with 4% paraformaldehyde for 10 minutes at room temperature to preserve cellular structures. To allow for antibody penetration, podocytes were treated with 0.2% Triton-X 100 in PBS for 10 minutes at room temperature. After washing with PBS, cells were blocked with 3% bovine serum albumin (BSA)-PBS for 30 minutes at room temperature to reduce non-specific antibody binding. Podocytes were incubated with a 1% BSA-PBS solution containing the primary antibody (Sigma Chemical Co., 1:200 dilution) at 4 ℃ overnight to allow for antibody binding to the target protein. after washing with PBS, cells were incubated with a fluorescein isothiocyanate (FITC)-labeled fluorescent secondary antibody (Cell Signaling Tech, 1:1,000 dilution) and 4’,6-diamidino-2-phenylindole (DAPI) staining solution (Guangzhou Wenqi Biotechnology Co., Ltd., Guangzhou, China) for 1 hour at room temperature, protected from light, to visualize the protein and cell nuclei, respectively. Podocytes were washed three times with PBS to remove unbound antibodies and staining solution. They were then sealed with an anti-quenching mounting medium to preserve fluorescence. Fluorescence images were captured using a Leica microscope (DM5000B) to visualize the distribution and intensity of protein expression.
Transmission electron microscopy (TEM) detection
The glomerular podocytes from the three groups were cultured, digested, and centrifuged to collect the cells. The podocytes were fixed in 2% paraformaldehyde for 1 hour at room temperature to preserve cellular structures. Following the initial fixation, cells were further fixed in 2% glutaraldehyde for an additional hour to enhance structural preservation. After fixation, cells were rinsed with PBS to remove any residual fixative, podocytes were then treated with 1% osmium tetroxide for 1 hour to enhance membrane contrast. Pre-staining with 1% uranyl acetate was performed for 1 hour to provide initial contrast to cellular structures. Podocytes were dehydrated through a graded ethanol series and embedded in Epon 812 resin. The resin was polymerized at 50 ℃ for 24 hours followed by 60 ℃ for 48 hours to solidify the embedding. Glass coverslips were treated with 49% hydrofluoric acid for 15 minutes to dissolve the glass, allowing for the removal of the resin blocks. The resin blocks were then trimmed and sectioned using an ordinary surgical blade to prepare thin sections suitable for TEM. The sections were post-stained with 2% uranyl acetate for 2 minutes to enhance contrast, followed by Reynolds’ lead citrate staining for 2 minutes to further improve the visualization of cellular structures. After the staining process, the sections were examined using TEM (Hitachi Co., Tokyo, Japan) to detect morphological and structural changes in the podocytes from each group.
Statistical analysis
All data were subjected to rigorous statistical analysis using IBM SPSS Statistics 22 software (IBM Corp., Armonk, NY, USA). Independent samples t-tests were employed to compare the means of two groups, and one-way analysis of variance (ANOVA) was utilized to evaluate differences across three or more groups. For all statistical tests, a two-sided P value threshold of less than 0.05 was set to determine statistical significance. To ensure the reliability of the findings, each experiment was conducted independently at least three times, yielding consistent results across replicates.
Ethical considerations
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study did not involve human or animal experiments, and individual informed consent was waived.
Results
Immunofluorescence analysis
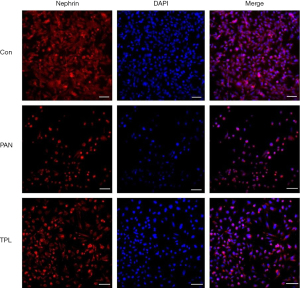
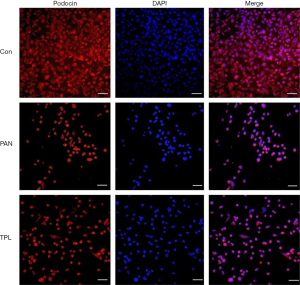
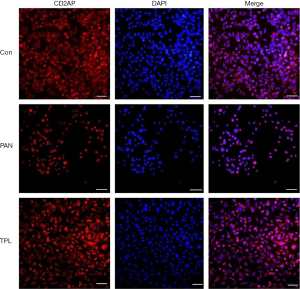
Immunofluorescence staining was performed to assess the expression and distribution of key podocyte proteins: nephrin, podocin, and CD2AP, in glomerular podocytes across different experimental groups. The results revealed significant differences in protein expression between groups. In the puromycin-induced injury group, the expression levels of nephrin, podocin, and CD2AP were markedly reduced compared to the normal control group. This indicates that puromycin treatment significantly compromised the expression of these crucial podocyte proteins. Conversely, treatment with TPL notably increased the expression of nephrin, podocin, and CD2AP in podocytes that were induced to injury by puromycin. This upregulation suggests a protective effect of TPL on podocyte protein expression. The differences in protein expression between the puromycin-induced injury group and the TP intervention group were statistically significant (P=0.04), as determined by independent samples t-tests, confirming the biological relevance of the observed changes. For a visual representation of these findings, refer to Figures 1-3, which illustrates the comparative expression and distribution of nephrin, podocin, and CD2AP among the groups.
TEM analysis
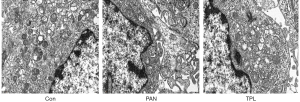
Our TEM analysis provided detailed insights into the structural integrity of mitochondria and autophagosomes in glomerular podocytes across different groups. In the normal control group, mitochondria were observed to be structurally intact and uniformly distributed, indicating healthy cellular function. This is consistent with the maintenance of normal physiological function in podocytes. The structure of autophagosomes, which are encapsulated by double-layer membranes, was clearly visible in normal podocytes. Autophagy is a critical process for cellular homeostasis, and the visibility of these structures suggests an active role in maintaining cellular health. In contrast, the PAN group showed significant deterioration in mitochondrial health, with increased swelling and disorganization of mitochondrial cristae, alongside a reduced number of autophagosomes. This indicates that puromycin treatment has a detrimental effect on podocyte structure and function. The TEM analysis revealed that TPL intervention significantly improved the mitochondrial swelling, arrangement of mitochondrial cristae, and the number of autophagosomes compared to the PAN group. This suggests a protective effect of TPL on podocyte health. For a detailed visual representation of these structural changes, refer to Figure 4, which contrasts the mitochondrial and autophagosomal structures between the normal control group and the PAN group, with and without TPL intervention. These results underscore the protective role of TPL in maintaining the structural integrity of mitochondria and autophagosomes in glomerular podocytes, which is crucial for understanding its therapeutic potential in renal diseases.
Discussion
Podocytes play a pivotal role in renal function, interdigitating and closely adhering to the basal lamina of glomerular capillaries. At the sites where these cells intersect, a specialized intercellular structure known as the lacunar septum is formed. This region is particularly rich in cell surface proteins, including nephrin, podocin, and CD2-associated proteins, which collectively envelop and safeguard the septum (10,11). The integrity of these structures is crucial for preserving the glomerular filtration barrier. Recent research has highlighted that dysregulation in the expression of key proteins such as nephrin, podocin, and CD2AP within the glomerular podocyte slit diaphragm (GPSD) is a primary contributor to proteinuria in several chronic kidney diseases (12-14). In the realm of nephrology, TPL has been clinically shown to effectively reduce proteinuria and protect renal function in various immune-mediated renal disorders (15,16). However, the literature does not yet elucidate whether the reduction in proteinuria is associated with the modulation of GPSD core protein expression. In this study, we found that puromycin-induced podocyte injury resulted in decreased expression of GPSD core proteins nephrin, podocin, and CD2AP, and that TPL intervention significantly up-regulated the expression of these podocyte proteins. This suggests that TPL may attenuate inflammation and modulate immune responses, potentially contributing to its protective effects on podocyte injury caused by puromycin.
Traditional Chinese medicine has a long history in the treatment of kidney diseases, and TPL has been widely used in the treatment of renal and immune disorders. Studies have shown that TPL exerts its pharmacological effects by regulating a variety of inflammatory cytokines and signaling pathways (17,18). In terms of renal protection, TPLs demonstrate their protective effects on the kidneys by interfering with inflammatory cytokines, the mTOR pathway, the p38 MAPK signaling pathway, the TLR4/NF-κB signaling pathway, and the RANK/RANKL signaling pathway, among others (19-21). Some studies have found that the protective effect of TPL on podocyte injury is associated with the regulation of the NF-κB signaling pathway. It has been confirmed that PM2.5 significantly increases the protein expressions of NF-κB/p65 and p-IκBα in podocytes, while treatment with TPL significantly reduces the expression of these proteins (9). Research has found that TPL effectively reduces proteinuria in puromycin nucleoside-induced renal damage in rats, an effect closely related to the improvement of foot process fusion, the reduction of desmin expression—a marker of podocyte injury—and the restoration of the expression and distribution of nephrin and podocin (22).
This study reveals the protective effects of TPL against PAN-induced podocyte injury through an in vitro model, particularly by upregulating slit diaphragm proteins (nephrin, podocin, and CD2AP) and stabilizing mitochondrial function. These findings not only provide new insights into the protective mechanisms of TPL but also offer a theoretical basis for its potential application in pediatric NS. The study employs a rigorous experimental design and statistical analysis, ensuring the reliability and reproducibility of the results. However, the study also has limitations. It is restricted to an in vitro model, which may not fully reflect the complex in vivo pathological environment. Additionally, the limited sample size, lack of long-term efficacy and safety assessments, and insufficient exploration of the specific molecular mechanisms and dose-response relationships of TPL are noted. Future research should further validate the protective effects of TPL in in vivo models and explore its potential applications in pediatric kidney diseases.
TPL demonstrates remarkable efficacy in the management of glomerulonephritis, particularly in reducing proteinuria levels (6,23). In the context of PAN-induced nephropathy, TPL has been shown to significantly lower proteinuria in both prophylactic and therapeutic mouse models (22). Clinical investigations have revealed that Tripterygium wilfordii effectively mitigates proteinuria in diabetic nephropathy (DN) patients, diminishes the accumulation of extracellular matrix (ECM) in the glomerular mesangial regions of diabetic animal models, and ameliorates renal histopathological alterations (24). Prior studies have suggested that the proteinuria-reducing effects of Tripterygium wilfordii are primarily associated with the preservation and restoration of the glomerular filtration barrier, although the precise mechanisms are still under investigation. Additionally, TPL has been found to effectively alleviate proteinuria in rats with PAN-induced renal injury, with its therapeutic actions closely tied to the improvement of podocyte effacement, decreased desmin expression, and the normalization of nephrin and podocin expression and distribution (22). In Henoch-Schönlein purpura nephritis (HSPN), a controlled clinical trial demonstrated that TPL significantly alleviated HSPN symptoms in pediatric patients (25). However, the application of TPL in clinical settings remains challenging due to its limited solubility and potential reproductive toxicity associated with long-term use (26,27). In this study, we observed the expression and changes of podocyte GPSD core proteins nephrin, podocin, and CD2AP, as well as changes in podocyte mitochondrial structure and function in a puromycin-induced podocyte injury model and after TPL intervention treatment, to further elucidate the molecular biological mechanism of TPL in the treatment of podocyte injury and proteinuria.
Conclusions
Glomerular podocytes, with their specialized structures, play a crucial role in maintaining the filtration barrier’s structure and function. Damage and loss of podocytes are primary causes of chronic kidney disease. The current study provides valuable information about the applications of TPL in podocyte injury. Future focus will be on exploring targeted therapies that TPL protects podocyte injury and mitigates mitochondrial dysfunction in podocytes, as well as investigating its role in other renal pathologies.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://pm.amegroups.com/article/view/10.21037/pm-24-73/rc
Data Sharing Statement: Available at https://pm.amegroups.com/article/view/10.21037/pm-24-73/dss
Peer Review File: Available at https://pm.amegroups.com/article/view/10.21037/pm-24-73/prf
Funding: This study was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pm.amegroups.com/article/view/10.21037/pm-24-73/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study did not involve human or animal experiments, and individual informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alenazi SA. Incidence and Pathological Patterns of Nephrotic Syndrome among Infants and Children: A Systematic Review. Cureus 2024;16:e58331. [Crossref] [PubMed]
- Finn LS. Nephrotic Syndrome Throughout Childhood: Diagnosing Podocytopathies From the Womb to the Dorm. Pediatr Dev Pathol 2024;27:426-58. [Crossref] [PubMed]
- Wannous H. Idiopathic nephrotic syndrome in Syrian children: clinicopathological spectrum, treatment, and outcomes. Pediatr Nephrol 2024;39:2413-22. [Crossref] [PubMed]
- Song J, He GN, Dai L. A comprehensive review on celastrol, triptolide and triptonide: Insights on their pharmacological activity, toxicity, combination therapy, new dosage form and novel drug delivery routes. Biomed Pharmacother 2023;162:114705. [Crossref] [PubMed]
- Ma R, Liu L, Liu X, et al. Triptolide markedly attenuates albuminuria and podocyte injury in an animal model of diabetic nephropathy. Exp Ther Med 2013;6:649-56. [Crossref] [PubMed]
- Liang D, Mai H, Ruan F, et al. The Efficacy of Triptolide in Preventing Diabetic Kidney Diseases: A Systematic Review and Meta-Analysis. Front Pharmacol 2021;12:728758. [Crossref] [PubMed]
- Wang L, Zhang L, Hou Q, et al. Triptolide attenuates proteinuria and podocyte apoptosis via inhibition of NF-κB/GADD45B. Sci Rep 2018;8:10843. [Crossref] [PubMed]
- Ren Q, Yu S, Zeng H, et al. The role of PTEN in puromycin aminonucleoside-induced podocyte injury. Int J Med Sci 2022;19:1451-9. [Crossref] [PubMed]
- Wan Q, Liu Z, Yang M, et al. Triptolide ameliorates fine particulate matter-induced podocytes injury via regulating NF-κB signaling pathway. BMC Mol Cell Biol 2020;21:4. [Crossref] [PubMed]
- Garg P. A Review of Podocyte Biology. Am J Nephrol 2018;47:3-13. [Crossref] [PubMed]
- Nagata M. Podocyte injury and its consequences. Kidney Int 2016;89:1221-30. [Crossref] [PubMed]
- Feng D. Phosphorylation of key podocyte proteins and the association with proteinuric kidney disease. Am J Physiol Renal Physiol 2020;319:F284-91. [Crossref] [PubMed]
- Kawachi H, Fukusumi Y. New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin Exp Nephrol 2020;24:193-204. [Crossref] [PubMed]
- Ren Q, You Yu S. CD2-associated protein participates in podocyte apoptosis via PI3K/Akt signaling pathway. J Recept Signal Transduct Res 2016;36:288-91. [Crossref] [PubMed]
- Jiang S, Wan F, Lian H, et al. Friend or foe? The dual role of triptolide in the liver, kidney, and heart. Biomed Pharmacother 2023;161:114470. [Crossref] [PubMed]
- Liu P, Zhang J, Wang Y, et al. The Active Compounds and Therapeutic Target of Tripterygium wilfordii Hook. f. in Attenuating Proteinuria in Diabetic Nephropathy: A Review. Front Med (Lausanne) 2021;8:747922. [Crossref] [PubMed]
- Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell 2007;130:769-74. [Crossref] [PubMed]
- Jiang M, Xie Y, Wang P, et al. Research Progress of Triptolide Against Fibrosis. Drug Des Devel Ther 2024;18:3255-66. [Crossref] [PubMed]
- Lv C, Cheng T, Zhang B, et al. Triptolide protects against podocyte injury in diabetic nephropathy by activating the Nrf2/HO-1 pathway and inhibiting the NLRP3 inflammasome pathway. Ren Fail 2023;45:2165103. [Crossref] [PubMed]
- Liang X, Chen B, Wang P, et al. Triptolide potentiates the cytoskeleton-stabilizing activity of cyclosporine A in glomerular podocytes via a GSK3β dependent mechanism. Am J Transl Res 2020;12:800-12. [PubMed]
- Ren L, Wan R, Chen Z, et al. Triptolide Alleviates Podocyte Epithelial-Mesenchymal Transition via Kindlin-2 and EMT-Related TGF-β/Smad Signaling Pathway in Diabetic Kidney Disease. Appl Biochem Biotechnol 2022;194:1000-12. [Crossref] [PubMed]
- Zheng CX, Chen ZH, Zeng CH, et al. Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Kidney Int 2008;74:596-612. [Crossref] [PubMed]
- Peng A, Gu Y, Lin SY. Herbal treatment for renal diseases. Ann Acad Med Singap 2005;34:44-51. [Crossref] [PubMed]
- Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 2008;4:39-45. [Crossref] [PubMed]
- Wu L, Mao J, Jin X, et al. Efficacy of triptolide for children with moderately severe Henoch-Schönlein purpura nephritis presenting with nephrotic range proteinuria: a prospective and controlled study in China. Biomed Res Int 2013;2013:292865. [Crossref] [PubMed]
- Tong L, Zhao Q, Datan E, et al. Triptolide: reflections on two decades of research and prospects for the future. Nat Prod Rep 2021;38:843-60. [Crossref] [PubMed]
- Noel P, Von Hoff DD, Saluja AK, et al. Triptolide and Its Derivatives as Cancer Therapies. Trends Pharmacol Sci 2019;40:327-41. [Crossref] [PubMed]
Cite this article as: Ren Q, Zhang D, Yu S. Protective effect of triptolide on puromycin-induced podocyte injury. Pediatr Med 2025;8:7.

