Abnormal uterine bleeding: a narrative review
Introduction
Onset of menstruation is a memorable event in all female adolescents. Menstrual cycles that become unpredictable, with presence of prolonged and excessive blood flow, can be appropriately concerning to an adolescent and their parent or caregiver. However, abnormal uterine bleeding (AUB) is a very common gynecological complaint, and often evaluation and management can be urgent. The purpose of this article is to review the prevalence and significance of AUB, discuss common etiologies, and evaluation and management approaches of AUB in the adolescent population. Additionally, we will review how to provide education to adolescents regarding AUB.
Methods
A literature search was performed on the electronic databases including MEDLINE and PubMed, from 1995 to 2019, to identify all relevant studies and reviews. A combination of the search terms included “abnormal uterine bleeding, menstrual, anemia, and adolescent”. The reference list also included studies identified manually, and studies referenced for other purposes.
Normal menstrual cycle
During adolescent development, menarche most often occurs within 3 years of thelarche, or breast budding, which is typically the first symptom of pubertal development in young women. Though there are variations across the globe, the median age of onset for menarche has remained stable, and occurs typically between ages 12–13 years in developed nations and well-nourished populations (1).
Menstrual cycles are often irregular during adolescence, particularly within the 2 years after their first cycle. Often young women have anovulatory cycles due to the immaturity of their hypothalamic-pituitary-ovarian (HPO) axis, causing relatively long cycles. By the third year after menarche, 60–80% of young women would have reached a typical adult cycle length with their cycles lasting 21–34 days from the first day of one period to the first day of the subsequent period (1). During first 2–3 years post-menarche, the cycle length may vary, and can range from 21 to 45 days. The duration of blood flow can range between 3 and 7 days with median blood loss between 30 and 40 milliliters. Counting number of soaked pads or tampons used per day is one way to estimate the menstrual blood loss; however, in adolescents this may not always be accurate. Typically, 6 or fewer soaked pads during menses is consistent with normal blood loss (2-4).
Definitions
AUB is the current preferred term by the International Federation of Gynecology and Obstetrics (FIGO) and is defined as “bleeding from the uterine corpus that is abnormal in duration, volume, frequency, and/or regularity and has been present for the majority of the previous six months” (5). Knowledge of normal parameters is important when identifying AUB based on the definition provided. Historically, non-specific and outdated terms such as “menorrhagia”, “dysfunctional uterine bleeding”, or “menometrorrhagia” have been used to describe “abnormal” uterine bleeding. For this reason, as well as the wide range of severity and etiologies for AUB, the FIGO system for nomenclature has been put in place to aid in the replacement of these terms with a standardized tool to measure frequency, duration, regularity, and flow volume of a woman’s menses (5).
Heavy menstrual bleeding (HMB) is currently defined by the FIGO to be a subjective excessive blood loss that interferes with the woman’s social, physical, material, and emotional quality of life (6). When quantified, HMB is defined by ≥80 mL of blood loss, estimated to be soaking a pad or tampon every 1–2 hours, or consistent clotting greater than the size of a quarter (1,7). In adolescent females, HMB is a relatively common complaint with current prevalence rates widely ranging from 12.1–37% (8-10). Disorders of menstruation not only have physical effects on women, but also impose psychological and social impacts on adolescent females with AUB.
Thus, it is important for health care providers (HCPs) to be aware of broader impacts on an adolescent patient’s quality of life. For this reason, during a comprehensive evaluation of adolescents who present with HMB, HCPs should be assessing involvement in sports and social activities, school performance, as well as attendance issues that the adolescent may be having in relation to her HMB (6).
Etiology
The second of two FIGO systems is the classification of the causes of abnormal bleeding during reproductive years; consisting of 9 categories and described using the acronym PALM-COEIN. These categories are Polyp; Adenomyosis; Leiomyoma; Malignancy and hyperplasia; Coagulopathy; Ovulatory Disorders; Endometrium; Iatrogenic; and Not otherwise classified. PALM categories define the structural causes of abnormal bleeding, while the COEIN categories separate non-structural causes (5). This popular classification is a useful guide for initial evaluation of AUB, however it is important to remember that the major causes of AUB in the adolescent population differ from those in the adult population.
While the FIGO system for nomenclature has helped with categorizing AUB for all pre- and post-menopausal women, it is important to identify if there are certain etiologies more commonly found in adolescent females. In adolescents, AUB is most commonly caused by non-structural causes, primarily immaturity of the HPO axis causing anovulatory cycles resulting in menstrual irregularities (1,6). Often, HMB related to HPO axis immaturity results in anovulatory cycles leading to longer, heavier menses when they do occur. According to endocrinological studies, 50% of menstrual cycles are ovulatory during the first 2 years after menarche, increasing to 75% at 5 years and eventually reaching a plateau of 80% (11). On average, menses become regular cycles 20 months post-menarche (7).
Bleeding disorders are also recognized as common causes for HMB in adolescents. A population-based study of almost 1,000 healthy adolescent females was done to assess for prevalence of HMB. In this study, almost 40% had self-identified HMB (defined as >6 pads or tampons in a 24-hour period). Of the 40% of adolescents who had experienced HMB, 20% reported that they had an underlying bleeding disorder (6).
Additionally, a retrospective study done by Alaqzam et al. looked at adolescent women under the age of 21 with identified HMB attending an adolescent hematologic/gynecologic clinic. From this group, 73 women were tested for a coagulopathy on the basis of medical history, after which 47% (n=34) of those women were formally diagnosed with a bleeding disorder [26 of the 34 diagnosed with von Willebrand Disease (VWD), and 8 of the 34 diagnosed with a platelet dysfunction (PFD)]. The remaining 53% (n=39) of women had HMB due to other causes such as anovulation (n=28), immune-mediated thrombocytopenic purpura (ITP) (n=6), or an unidentified cause (n=5) (7).
A high prevalence of VWD and PFD were found in adolescent women with identified HMB. Data is relatively limited regarding the prevalence of bleeding disorders among adolescents with HMB, but currently suggests that the prevalence of coagulopathies in adolescents is at least as likely as it is in adult women with HMB (12).
Other common presentations include amenorrhea greater than 90 days between menses (even for one cycle), and intermenstrual bleeding (IMB) that can occur secondary to rapid weight changes, stress, or endocrinopathies (1). Less likely causes of AUB in the adolescent population include structural causes and endometrial cancer. Structural causes are responsible for less than 2% of AUB in the adolescent population (6,11). Endometrial cancer risk in patients younger than 20 years old is 0.2 per 100,000 women (13).
Evaluation
The clinical presentation of AUB in adolescents is most often subjective, self-identified, and reported by patients or their caregivers. One’s perception may differ in terms of amount of menstrual bleeding that is soaking through pads or tampons, and how often these should be changed. In extreme cases, the patient or caregiver reports experiencing symptoms indicative of significant blood loss resulting in symptomatic anemia, such as fatigue, dizziness, and pallor that are more pronounced during menses.
When evaluating AUB, we must consider that AUB can be identified by HMB (AUB/HMB) or IMB (AUB/IMB) and can be classified as either acute or chronic. It can also be caused by a variety of etiologies; ranging from structural abnormalities, disorders of hemostasis, dysregulation of endocrine signaling, or premalignant disease. Table 1 summarizes the differential diagnosis in evaluating causes for AUB.
Table 1
| Categories | Conditions |
|---|---|
| Endocrine | Immaturity of the hypothalamic-pituitary-ovarian axis |
| Polycystic ovary syndrome | |
| Congenital adrenal hyperplasia | |
| Thyroid disease | |
| Primary pituitary disease | |
| Coagulopathy | von Willebrand disease |
| Platelet dysfunction | |
| Clotting factor deficiency | |
| Thrombocytopenia | |
| Pregnancy | Ectopic pregnancy |
| Abortion | |
| Gestational trophoblastic disease | |
| Infection | Adenomyosis |
| Cervicitis | |
| Medication | Anticoagulants |
| Hormonal contraceptives | |
| Uterine lesions | Polyp |
| Leiomyoma | |
| Intrauterine device | |
| Malignancy | Estrogen producing ovarian tumors |
| Androgen producing ovarian tumors | |
| Rhabdomyosarcoma | |
| Iatrogenic | Secondary to radiation or chemotherapy |
| Other | Foreign body |
AUB, abnormal uterine bleeding.
Workup and diagnosis of AUB in adolescents should focus heavily on the history and physical exam. It is imperative that the patient history is taken with and without parents/guardians present to allow for open communication and confidentiality. A thorough menstrual history should include age of menarche, menstrual regularity, duration, flow and number of pads/tampons used per day, and first day of last menstrual period (LMP). The number of pads/tampons per day allows for objective quantification of menstrual flow (Figure 1) (14).
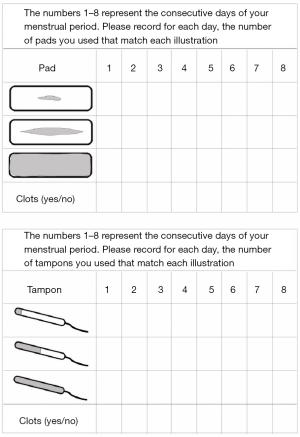
Another tool for quantifying blood loss during menses is a pictorial bleeding assessment chart with different versions available online. This chart allows the adolescent to visually identify the saturation of female hygiene products. It may also be beneficial for the patient to complete a menstrual journal that includes associated symptomatology over a period of time in order to establish a pattern or lack thereof. Smart phone applications are also available and may be a convenient way for an adolescent to track menstrual cycles.
Clinicians should obtain relevant review of systems including constitutional symptoms (appetite change, weight change, fatigue, change in activity), symptoms relating to any endocrinopathies (heat/cold intolerance, hirsutism), infectious diseases (fever, rash), and hematologic disorders (gum bleeding, prolonged bleeding after minor injury, easy bruising). Additionally, past medical and surgical history, family history (menstrual history of female family members, bleeding or clotting disorders), social history (diet/nutrition, exercise, sexual history), and current medications (short-term prescriptions, vitamins/supplements, contraceptives) should be reviewed.
In the majority of cases, sexual history should be obtained confidentially. The adolescent should be asked about coitarche, current and last sexual activity, number of partners, sex or gender of partners, nature of sexual activity (oral, vaginal, or anal), contraception method including barrier methods and use of emergency contraception and history of treated or untreated sexually transmitted infections (STI). Adolescent patients may be unwilling to answer or admit to sexual activity in the presence of parents/guardians, therefore reassuring confidentiality is crucial while taking a separate history with the patient alone.
Physical exam should be broad, covering all systems, including vital signs (hemodynamic stability), general appearance (body habitus, fatigue, distress), endocrine (thyroid/goiter, neuropathy, acanthosis nigricans, acne, facial and body hair distribution, stretch marks, moon facies, or buffalo hump), hematologic (pallor, bruising, rash, lymph nodes), external genitalia (secondary sex characteristics), and speculum exam in select cases. Basic organ systems must also be covered including cardiac, pulmonary, gastrointestinal, and psychiatric exams.
Laboratory testing should include the following studies at minimum: beta-human chorionic gonadotropin (beta-hCG) and complete blood count (CBC). Additional workup may include blood glucose, thyroid studies, peripheral blood smear, iron studies, and STI testing (in sexually active patients), as guided by history, physical exam findings, and differential diagnosis. In patients with family history or personal history of clotting or bleeding disorders including HMB, testing a von Willebrand (vW) profile is justifiable because adolescents with HMB are 10–20 times more likely to have VWD. The profile includes testing for vW factor (vWF) antigen and activity, multimer analysis and factor VIII levels (7).
External pelvic imaging may be warranted in cases where structural abnormality is suspected and if speculum exam and/or transvaginal ultrasound is not desired or possible. It is important to know the LMP prior to ordering pelvic ultrasound, otherwise endometrial thickness without correlation to the specific phase of the menstrual cycle may not be useful. For patients with risk factors for endometrial cancer, such as obesity and at least 2–3 years of untreated AUB, it is reasonable to obtain an endometrial biopsy when other causes of AUB have been ruled out (7,15).
Management
The management approaches to AUB are dependent on the underlying etiology and severity of bleeding. For AUB not caused by an underlying medical disorder, i.e., anovulatory bleeding, the mainstay of treatment includes combined oral contraceptives (COCs) and nonsteroidal anti-inflammatory drugs (NSAIDs) for pain relief. Depending on hemoglobin levels, iron may or may not be added as a supplement (11). Table 2 summarizes the classification based on severity and recommended treatment approaches as discussed below.
Table 2
| Classification | Severity | Treatment |
|---|---|---|
| Mild | Light to moderate bleeding and normal to slightly decreased hemoglobin ≥12 g/dL | Observation |
| NSAIDs | ||
| Menstrual journal | ||
| +/− oral iron supplementation | ||
| Moderate | Moderate to heavy bleeding and hemoglobin ≥10 g/dL | Monophasic COCs |
| Progestins* | ||
| NSAIDs | ||
| Oral iron supplementation | ||
| Severe | Heavy bleeding and hemoglobin <10, or <7 g/dL without active bleeding | Hospitalization |
| IV estrogen and COCs (taper dosing) | ||
| Progestins* | ||
| IV/oral iron supplementation | ||
| If refractory: mechanical/surgical interventions |
*, if patient has contraindications to estrogen. NSAIDs, nonsteroidal anti-inflammatory drugs; COCs, combined oral contraceptives.
Mild bleeding
The majority of anovulatory uterine bleeding can be managed on an outpatient basis. For mild cases of anovulatory bleeding with hemoglobin >12 g/dL, observation and NSAIDs are widely accepted as the treatment plan. The patient should be encouraged to keep a menstrual journal, ideally before and after treatment, to document efficacy or failure of treatment. In patients with mild anovulatory bleeding with hemoglobin between 10–12 g/dL, iron supplementation (usually 60 mg daily) may be added to prevent symptomatic anemia. Patients may also opt to increase dietary iron if supplementation is not desired (11,13).
Moderate bleeding
For moderate cases of anovulatory bleeding treatment, iron supplementation and hormonal therapy, specifically monophasic COCs are recommended. In anemic patients with acute bleeding, low-dose COCs containing 20–35 mcg of ethinyl estradiol (EE) can be given every 8–12 hours until bleeding ceases, then the same pill can be continued for 21 days followed by 7 days of placebo or medication pause (11,13).
If bleeding restarts while on initial COC treatment, the pill may be taken twice daily for 21 days total followed by 7 days of placebo or medication pause. Higher amounts of estrogen can cause nausea, so it is recommended to provide a prescription for antiemetics while on the twice daily dose of COCs. The COC prescription should be continued for 3–6 months until hemoglobin level reaches at least 12 g/dL (11,13).
In patients who have contraindications to taking estrogen or those who are not actively bleeding, oral progestin given for 12 days per month followed by a withdrawal bleed 2–7 days after the 12th day can be an effective alternative treatment (11,13).
Severe bleeding
In patients with severe anovulatory bleeding classified by hemoglobin level <7 or <10 g/dL and are symptomatic with active bleeding, hospitalization may be required for a more aggressive treatment approach. Patients who are asymptomatic, hemodynamically stable with a hemoglobin level of 8–10 g/dL, and who are reliable to return for follow-up may be treated in the outpatient setting with monophasic COC (30–50 µg EE) taper and iron supplementation (60–120 mg).
As stated before, antiemetics should be provided to treat nausea/vomiting from the higher doses of EE. An example of a COC taper is taking 1 pill every 6 hours for 2–4 days, then 1 pill every 8 hours for 3 days, then 1 pill every 12 hours for 14 days. Again, follow-up in the outpatient setting is essential to prevent recurrence and address efficacy (11,13).
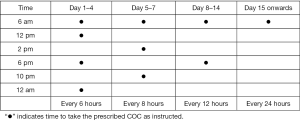
Hospitalization is necessary in cases of severe bleeding when the patient is hemodynamically unstable or symptomatic. Treatment involves a COC taper similar to the one presented above with additional levels of tapering. For example, take 1 COC every 4 hours until bleeding slows, then gradually taper to every 6, 8 and then 12 hours. COCs should be continued for an additional 21 days or until hemoglobin level is above 10 g/dL. Figure 2 demonstrates this taper dosing that the patient can use as a guide outpatient.
If treatment fails in the first 24 hours and the patient continues to bleed heavily, or if patient is unable to take oral medications, intravenous conjugated estrogen (25 mg) can be given every 4–6 hours for 24 hours transitioning to the COC taper after bleeding has slowed/ceased. Other medications to be considered in refractory bleeding include medroxyprogesterone acetate 20 mg orally 3 times per day for 7 days, or tranexamic acid, a commonly used antifibrinolytic in trauma and surgical patients, given orally or intravenously, 3 times per day for 5 days (15).
Mechanical intrauterine tamponade with a Foley catheter may also be used to control bleeding that has failed medical treatment. Surgery is the most drastic treatment for unrelenting bleeding and should only be considered for the most refractory cases due to potential problems, including intraoperative complications and future infertility. It is important to note that adolescents who require hospitalization or blood transfusion for heavy bleeding have a 20–30% risk of coagulopathy. VWD is usually the most common etiology in these women with likely a bleeding disorder (13).
Patient education
It is recommended that HCPs begin anticipatory guidance with respect to pubertal development and menstrual cycles before the child reaches these milestones, usually beginning around the ages of 7 and 8 years. The young adolescent should be educated on the normal ranges of acceptable variations in patterns of menstrual bleeding. Providing expectations to adolescents and their parents/guardians about what is considered “abnormal” or “irregular” bleeding is key to preventing unnecessary anxiety and worry. The adolescent should be asked about their menstrual patterns, with accurate documentation of the first day of LMP especially during wellness visits (1). It is important to remind the adolescent female that menstrual cycles become regular in 60–80% of young females by the third year after menarche (1,11).
AUB is one of the most common gynecological complaints that brings adolescent patients to the emergency department. Such an experience may cause anxiety and costs money and time. Patients that feel supported by their physician and office staff will be more likely to contact their HCPs with questions prior to making a trip to the urgent care or the emergency department. It can be beneficial for the young adolescent to chart her menses when menstrual history is vague, or if unable to recall accurate data.
The psychological effects of AUB specific to adolescents include negative impact on quality of life secondary to missing school or social events, fatigue from anemia, and frustration with constant menstrual hygiene. It is important to give emotional support to the young adolescent and in return, establish a strong physician-patient relationship that will facilitate more open and honest conversations in the future.
Conclusions
AUB is a common gynecological complaint in the adolescent population and warrants appropriate evaluation and treatment. A thorough history with complete physical exam, followed by appropriate testing should be obtained in a timely fashion. AUB can be identified by HMB (AUB/HMB) or IMB (AUB/IMB) and can be classified as either acute or chronic. AUB can also be caused by a variety of etiologies and in adolescents, most commonly is caused by immaturity of the HPO axis causing anovulatory cycles and menstrual irregularities. The differential for AUB is broad, and both severity and acuity of the underlying cause dictate treatment approach. The best preventive measure is education of the patient and caregivers on monitoring menstrual cycles and knowing when help is necessary.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Pediatric Medicine for the series “Adolescent Gynecology”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.06.11). The series “Adolescent Gynecology” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- ACOG Committee Opinion No. 651: Menstruation in Girls and Adolescents: Using the Menstrual Cycle as a Vital Sign. Obstet Gynecol 2015;126:e143-6. [PubMed]
- National Health and Nutrition Examination Reproductive Health Dataset. Accessed 3 June 2019. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/RHQ_I.htm
- Upadhya KK, Sucato GS. Menstrual problems. In: Kliegman R, St. Geme J. editors. Nelson Textbook of Pediatrics. Philadelphia: Elsevier, 21st edition, 2019:1058-9.
- Greydanus DE, Sorrel S, Omar HA. Menstrual disorders in the female adolescent. In: Greydanus DE, Patel DR, Omar HA, et al. editors. Adolescent Medicine: Pharmacotherapeutics in General, Mental and Sexual Health. Berlin: Walter de Gruyter, 2012:301-30.
- Munro MG. Practical aspects of the two FIGO systems for management of abnormal uterine bleeding in the reproductive years. Best Pract Res Clin Obstet Gynaecol 2017;40:3-22. [Crossref] [PubMed]
- Haamid F, Sass AE, Dietrich JE. Heavy Menstrual Bleeding in Adolescents. J Pediatr Adolesc Gynecol 2017;30:335-40. [Crossref] [PubMed]
- Alaqzam TS, Stanley AC, Simpson PM, et al. Treatment Modalities in Adolescents Who Present with Heavy Menstrual Bleeding. J Pediatr Adolesc Gynecol 2018;31:451-8. [Crossref] [PubMed]
- Friberg B, Ornö AK, Lindgren A, et al. Bleeding disorders among young women: a population-based prevalence study. Acta Obstet Gynecol Scand 2006;85:200-6. [Crossref] [PubMed]
- Barr F, Brabin L, Agbaje S, et al. Reducing iron deficiency anaemia due to heavy menstrual blood loss in Nigerian rural adolescents. Public Health Nutr 1998;1:249-57. [Crossref] [PubMed]
- James AH. Women and bleeding disorders. Haemophilia 2010;16:160-7. [Crossref] [PubMed]
- Mullins TL, Miller RJ, Mullins ES. Evaluation and Management of Adolescents with Abnormal Uterine Bleeding. Pediatr Ann 2015;44:e218-22. [Crossref] [PubMed]
- Elmaoğulları S, Aycan Z. Abnormal Uterine Bleeding in Adolescents. J Clin Res Pediatr Endocrinol 2018;10:191-7. [Crossref] [PubMed]
- Committee on Practice Bulletins—Gynecology. Practice bulletin no. 136: management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol 2013;122:176-85. [Crossref] [PubMed]
- Heavy menstrual bleeding. CDC. Accessed 5 June 2019. Available online: https://www.cdc.gov/ncbddd/blooddisorders/women/menorrhagia.html
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: Management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol 2013;121:891-6. [Crossref] [PubMed]
Cite this article as: Miller K, Konal J, Brown K, Cabral MD. Abnormal uterine bleeding: a narrative review. Pediatr Med 2019;2:27.