Pectus excavatum in adolescents and children: the Nuss technique
Introduction
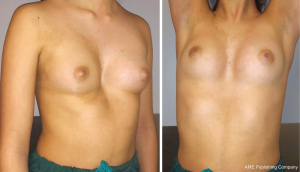
Pectus excavatum (PE), or funnel chest, is one of the most common anterior chest wall deformity, happening about 1 in every 300–1,000 births (1). PE has an incidence five times higher in males than females (2). Approximately half of these children have at least one family member with other thoracic abnormalities. PE is normally identified during infancy, after it slowly progresses during growth and begins more evident during puberty (3). The spontaneous regression is not frequent, and physical activity does not resolve these abnormalities. The anteroposterior diameter of chest of PE patients is decreased and almost half of them show a deeper concavity on one side (Figure 1).
On the contrary, the other half has a symmetric depression which involves the low sternum, and laterally the costochondral junctions.
The severity of PE usually increases with deep inspiration, such phenomenon is termed paradoxical breathing. To date, the pathogenesis of this disease remains unclear. The deformity might depend on an unbalanced growth of the costochondral regions thus explaining the frequent appearance in patients of an asymmetric PE and the presence of wall deformities also in their relatives (3-5).
Involved cartilages are often deformed, rotated and fuse together, and the xiphoid is frequently bifid, crooked, elongated or deviated to one side. Often, PE affected patients hold a hunched posture and have a bulging abdomen. Moreover, these children could be affected by musculoskeletal abnormalities, including scoliosis (4), and other heritable disorders of the connective tissue, such as Marfan syndrome, Ehlers-Danlos syndrome, Poland disease, Mitral valve prolapse or MASS phenotype (3,5,6). Interestingly, about fifty per cent of Marfan syndrome affected patients have a concurrent PE (6).
Symptoms
During early childhood, patients affected by PE are usually asymptomatic. Differently, symptoms like easy tiredness and decreased physical endurance could appear during adolescent age or during sport-correlated physical effort. Moreover, patients may experience a sharp chest pain and/or discomfort in the anterior chest during physical exercise (2,5).
In a quarter of patient with PE may appear common symptoms such as tachycardia, palpitations, and exercise-induced wheezing, furthermore they show an increased susceptibility of respiratory infections. Children with very-severe chest deformity frequently have cardiopulmonary impairment in part due to a sternum compression on the heart and in part to a reduced pulmonary expansion, as confirmed by the “restrictive pattern” assessed using the Pulmonary Function Test (PFTs) exam (2,5,7-10).
Often, pediatricians do not correctly inform family of patients regarding the physiological effects of the deformity and regarding the availability of a safely and highly performant surgical correction (2).
Fortunately, nowadays patients have the opportunity to inform themselves via internet about thoracic deformities and their treatment. Moreover, they have the opportunity to reach other patients and knowledgeable physicians.
Perioperative studies
Radiological preoperative assessment of PE patients is based on computed tomography (CT) scan or magnetic resonance (MR) scan. CT scan allows to provide some chest indices (i.e., Correction Index, Asymmetry Index and the Haller Index) and other helpful information such as the displacement, rotation and compression of the heart, especially in cases with significant asymmetry (9,11). Perioperative assessment may include the 3D optical evaluation thus being extremely useful for those patients who are conservatively treated (12).
Cardiac function and morphology may be easily assessed by electrocardiogram and echocardiography. The latter may be especially relevant to measure the aortic root diameter when Marfan syndrome is suspected. In these circumstances, is recommended a consultation with both pediatric cardiologist and geneticist before surgery (6).
Standard PFTs is recommended to evaluate pulmonary volume, pulmonary capacity, and exercise tolerance, whereas the routine blood test is used to rule out coagulation disorders (8,10-12). Skin allergy to metals should be tested, because bar and stabilizer are stainless-steel devices. In case of hypersensitivity to Nickel sulphate a titanium-implant have to be requested.
Surgical procedure
The minimally invasive procedure for PE repair (MIRPE), diffused by Donald Nuss in 1998 (10), is a worldwide methodology adopted by pediatric, thoracic, and plastic surgeons (10,13).
The Nuss technique does not involve the resection of costal cartilages or the sternal fracture but employ one or more metal bars to relocate the sternum into the right position (3,10,11,13). Initially, Nuss developed such technique working on children, whose flexible chest wall allows an easier sternum uplift (10).
From 1998, worldwide medical centers reported their experience with the Nuss procedure introducing numerous modifications to achieve higher security and efficacy in bar placement and removal (11,13,14). When the Nuss procedure became a standard treatment, surgical indications were expanded (10-14).
Promising preclinical studies was performed in our institute in the attempt to use a partially resorbable matrices, with the aim of reducing systemic toxicity from metal implant as well as osteo-integration or to use a sensorized bar for a patient-specific treatment time (15).
Patient selection
During these years, the most significant change was made extending the age selection criteria for PE surgery. In the initial report, the surgery was undertaken in preschool children with a median age of 5 years and nobody of them was older than 15 years (10).
As the experience with MIRPE matured, the indication of the procedure was postponed in an age near to the pubertal spurt when the chest is almost shaped, but still moldable (3).
Currently, surgeons prefer operating teenager patients. Indeed, numerous authors elect a mean age of 14 years old, resulting in an increased 25th percentile of 13 years old (16). On the other hand, other authors report outstanding outcomes in chest repair of patients as young as toddlers (17). Reports of acquired thoracic dystrophy in young children undergoing Ravitch procedures also drove the age of referral higher (18). Our view supports the evolution of Nuss thought, in fact we share the therapeutic plan with the teenage patient and his relatives, but both have to demonstrate a strong will to undergo the procedure. The surgical technique herein presented is an evolution of the Nuss procedure which is routinely performed at our Institute.
Operative technique
Minimally invasive repair of PE
The patient is placed in the supine position on the operating table. The chest must be elevated, using a pillow to allow the arms to be adducted in an inferior position. It is necessary that the child is intubated with a single-lumen tracheal tube and ventilated with low volumes. The most depressed area of the sternal plate and the hinge bilateral points are identified and marked. The length of the bar have to cover the anterior part of patient chest. The bar is asymmetrically bended with a manual device over a moldable template so as enhance contrivance stability (19). A 5-mm thoracic port is introduced in a lateroposterior right position and carbon dioxide is insufflated at 4 to 6 mm Hg pressure to partly reduce lung expansion and producing an operative space. This procedure allow a clear sight of the mediastinum. A stainless steel wire is passed through the sternum transversely and in combination with a table lift allows the chest to be raised.
Two small lateral incisions (3 to 4 cm long) are bilaterally made just at the inferior edge of pectoralis major muscle. After that, subcutaneous planes are created on both sides. Pectoralis major bundles are released from the costal insertions creating a submuscular passage. Afterwards, an introducer is inserted into the right chest in the selected intercostal space, and under-vision dissection is performed above the pericardium. This step is facilitated by the elevation of the depressed sternum. Furthermore, small laceration produced during the smooth dissection facilitate the gas diffusion in the left pleura.
Once the corresponding left intercostal space is reached, the introducer tip is pushed out and connected to a side of a one 40 cm long polyvinyl chloride suction connecting tube (Extrudan Surgery, Birkerod, Denmark). The other side of the tube is connected to the right end of the curved bar. The introducer is carefully pulled backward from left to right dragging the tube and forming a trail, which allows the bar to pass with its concave side up (18-21).
In few seconds the entire procedure is easily completed under thoracoscopic vision. Finally, the bar is rotated 180 degrees around its long axis pushing up the sternum. Once the bar is in place, it is necessary placing one stabilizer to maximize its mechanical stability.
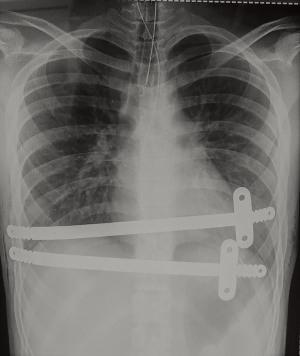
Usually a second bar with stabilizer is placed in the upper or lower intercostal space in the same way described above.
The induced pneumothorax is aspirated and usually the patient brings back to the ward.
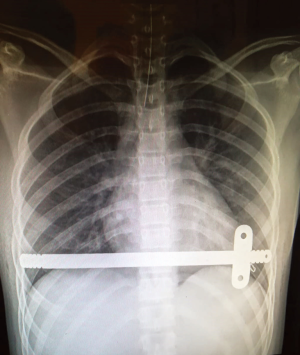
Progressive mobilization is encouraged, but avoiding any chest torsion. The patients are usually discharged after 5–6 days of hospitalization, after at least one chest radiological control was performed. Two examples of post operatory chest X-ray we performed are shown in Figures 2,3.
Patients are usually followed up during the post-operative period with outpatient check at 1, 6 and 18 months (19-21).
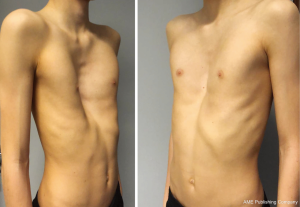
An example of Boy with Pectus Excavatum are shown in Figure 4.
Implant removal procedure
The procedure takes place usually 2–3 years after MIRPE. The patient is placed another time in the supine position with the chest raised, as previous reported. Thoracic scars are bilaterally excised. The terminal portions of the bars with their stabilizers are localized and freed using diathermy. In patients where bar ends are not palpable, a perioperative ultrasound scan may be performed few minutes before the surgery. This operation is necessary to label the actual projection on the skin of the metal plate marking it with a felt-tip pen (19).
Once freed, the stabilizers are either removed from the bar or removed together with the bar itself in a second time. A hook is inserted into the hole at the end of the bar to allow it to be pulled out. In case of callus formation around the bar, hammer and chisel are employed to remove them. If the bar is extremely bent, the flipper tool is used to straighten the bar before its removal. For these patients who had a residual chest wall defect at time of implant removal, we have proposed autologous fat grafting at the same operative session. The donor areas are abdomen, trochanteric region, and inner thigh. The tissue is gently collected through a thin cannula, filtrated, and promptly injected into the residual defect (20). The patient usually returns to the ward after the procedure and is discharged on the first post-operative day.
Discussion
Nowadays the treatment of choice for symptomatic pectus excavatum (PE) in children is the Nuss procedure. Introduced in 1987 (10,11), it has gained popularity after the publication of the 10-year experience (10,11) and reached worldwide diffusion secondary to the use of thoracoscopy and the conception of new and specific instrumentations. With the introduction of thoracoscopy, MIRPE has reached an extremely lower rate intraoperative complication, lower incidence of major complication and a post-operative mortality very close to zero (14). Hebra and co-workers (22) performed a survey between the Chest Wall International Group (CWIG) members about morbidity and mortality during MIRPE. Furthermore, they combined these data with the information obtained from a systematic review of the literature: out of a total of around 11,000 treated cases the authors identified 32 and 27 cases of un-reported and reported cases of life-threatening complications. Within these cases the authors recognized 7 and 4 un-published and published Nuss procedure related death (22). Intra-operative complications are extremely rare and also post-operative complications are infrequent and often classified as minor (14).
Post-operative complications can be resume as early and late adverse events. The most frequent complication is small pneumothorax not requiring chest tube (range 66%) and it is due to incomplete removal of the capnothorax or partial re-expansion of the lung and resolves spontaneously (11). Large pneumothorax is more frequent in redo patients and are caused by small air leak secondary to lysis of adhesions (23,24). Wound infections occur in less than 1–2% of patients and the site of stabilizers is more frequently involved (25-27). Generally, antibiotic treatment and wound drainage are necessary and resolutive (26). Bar infection and bar symptomatic displacement are the two causes of early bar removal. The incidence of bar displacement was higher in the first era of MIRPE, but this event dropped down significantly with the introduction of the stabilizers (5,11,14). Pericarditis, chronical pleural effusion or wound infection can develop months after surgery and could be secondary to nickel allergy (28).
Post-bar removal recurrence ranged between 0% to 33%; large and more recent studies demonstrated a low recurrence rate, close to 0% (10,13,14,29). It is well-established that the recurrence rate increases inversely with the bar keeping time: if the bar is removed before 24 months the risk of recurrence can reach the 40% of patient treated (30,31). Another variable that can affect the recurrence rate is the age at the time of MIRPE. Patients treated too early, completed MIRPE before the pubertal growth, PE associated with Marfan syndrome or could not keep the bar in situ for the standard time have an increased risk of recurrence (10,13,14,28,31).
Severe deformity is associated with a restrictive pattern on pulmonary function tests and with impaired cardiac function, but some papers demonstrated a positive change on PFTs and cardiac function. Before surgery, PE patients show a low forced vital capacity (FVC) and exercise limitation in comparison to the control group. However, MIRPE significantly improves exercise tolerance and oxygenation (11,32,33). On the other hand, Lawson et al. did not report significant positive changes in FVC or FEV1 tested after MIRPE procedure in children, conversely significant improvement on the same tests were observed in adolescents (28,34). Instead regarding cardiac function several authors showed an association between physiological impairment and weak exercise capacity with a reduced cardiovascular performance (11,34,35-38). Coln and colleagues tested children cardiac function with echocardiography/EKG with exercise before surgery and after MIRPE, demonstrating the decreased incidence of arrhythmias and in 93% of patients the disappearance of cardiac compression correlated symptoms (35). Heitzer and Wollschlager in children with severe deformity reported the effect of sternum’s mechanical compression on the heart, using catheterization, CT scan and ECG (39). The compression on the heart causes a diminished stroke volume (SV), as well as cardiac output (CO) and cardiac index (CI), and combined with a lung restriction PTFs leads to a depression cardiopulmonary function (11,33,35,36). The harvesting of objective outcomes of MIRPE derived from radiological data is not feasible for the high costs of MRI and for the radiation exposure of CT-scans. Thus, patients’ satisfaction, cosmetic outcomes and personal surgeon judgement are the only data achievable regarding long term outcomes. In the large experience published (40), anatomical long-term outcomes evaluated by surgeons who performed MIRPE demonstrated excellent results in over 85% of patients, good in 10% and only in 1.4% the MIRPE failed. Comparable results, ranged between 76–100%, were also reported by other authors (10,13,14,22,23,28,31,32).
To establish the satisfaction degree after MIRPE, several studies analysed data obtained from scales or questionnaires administered directly to patients or their parents (40,41). Some studies reported an 85–100% satisfactory cosmetic outcome (28,40).
Specific questionnaires were developed to objectively assess the improvement of self-esteem: Single Step Questionnaire (SSQ), Pectus Excavatum Evaluation Questionnaire (PEEQ); Child Health Questionnaire (CHQ-CF87). All paper published showed a high percentage of patients (96–100%) who had an improvement of self-esteem (28,40,42,43).
These questionnaires evaluated some component: psychosocial as depression or irritability; social self-consciousness as reluctance to undress in public; physical as chest pain or asthenia (28,42,43). Regardless of the type of survey used, MIRPE is associated with positive changes in social life and social behaviour and for these reasons some authors justify the use of surgery for cosmetic reasons in children or in adolescent (10,11,28,42,44).
In conclusion, MIRPE is certainly safe and effective in the treatment of PE in children and can be proposed also for complex deformities. We can assess MIRPE is the procedure of choice for symptomatic or cosmetic PE in children for the objective improvement of cardio-pulmonary function and the high rate of patients’ satisfaction.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Paolo Scanagatta) for the series “Pediatric Thoracic Surgery” published in Pediatric Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.07.02). The series “Pediatric Thoracic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Molik KA, Engum SA, Rescorla FJ, et al. Pectus excavatum repair: experience with standard and minimal invasive techniques. J Pediatr Surg 2001;36:324-8. [Crossref] [PubMed]
- Shamberger RC. Congenital chest wall deformities. Curr Probl Surg 1996;33:469-542. [Crossref] [PubMed]
- Nuss D, Kelly RE Jr. Chest wall deformities. Semin Pediatr Surg 2008;17:153. [Crossref] [PubMed]
- Ghionzoli M, Martin A, Bongini M, et al. Scoliosis and Pectus Excavatum in Adolescents: Does the Nuss Procedure Affect the Scoliotic Curvature? J Laparoendosc Adv Surg Tech A 2016;26:734-9. [Crossref] [PubMed]
- Goretsky MJ, Kelly RE, Croitoru D, et al. Chest wall anomalies: pectus excavatum and pectus carinatum. Adolesc Med Clin 2004;15:455-71. [Crossref] [PubMed]
- Tocchioni F, Ghionzoli M, Messineo A, et al. Pectus excavatum and heritable disorders of the connective tissue. Pediatr Rep 2013;5:e15 [Crossref] [PubMed]
- Malek MH, Fonkalsrud EW, Cooper CB. Ventilatory and cardiovascular responses to exercise in patients with pectus excavatum. Chest 2003;124:870-82. [Crossref] [PubMed]
- Maagaard M, Heiberg J. Improved cardiac function and exercise capacity following correction of pectus excavatum: a review of current literature. Ann Cardiothorac Surg 2016;5:485-92. [Crossref] [PubMed]
- Haller JA, Kramer SS, Lietman SA, et al. Use of CT scan for selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg 1987;22:904-6. [Crossref] [PubMed]
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. [Crossref] [PubMed]
- Nuss D, Kelly RE. Indications and technique of Nuss procedure for pectus excavatum. Thorac Surg Clin 2010;20:583-97. [Crossref] [PubMed]
- Uccheddu F, Ghionzoli M, Volpe Y, et al. A Novel Objective Approach to the External Measurement of Pectus Excavatum Severity by Means of an Optical Device. Ann Thorac Surg 2018;106:221-7. [Crossref] [PubMed]
- Kelly RE, Goretsky MJ, Obermeyer R, et al. Twenty-One Years of Experience With Minimally Invasive Repair of Pectus Excavatum by the Nuss Procedure in 1215 Patients. Ann Surg 2010;252:1072-81. [Crossref] [PubMed]
- Park HJ, Lee SY, Lee CS, et al. The Nuss procedure for pectus excavatum: evolution of techniques and early results on 322 patients. Ann Thorac Surg. 2004;77:289-295. [Crossref] [PubMed]
- Betti S, Ciuti G, Ricotti L, et al. A sensorized Nuss bar for patient-specific treatment of Pectus Excavatum. Sensors 2014;14:18096-113. [Crossref] [PubMed]
- Papandria D, Arlikar J, Sacco Casamassima MG, et al. Increasing age at time of pectus excavatum repair in children: emerging consensus? J Pediatr Surg 2013;48:191-6. [Crossref] [PubMed]
- Park HJ, Sung SW, Park JK, et al. How early can we repair pectus excavatum: the earlier the better? Eur J Cardiothorac Surg 2012;42:667-72. [Crossref] [PubMed]
- Weber TR, Kurkchubasche AG. Operative management of asphyxiating thoracic dystrophy after pectus repair. J Pediatr Surg 1998;33:262-5. [Crossref] [PubMed]
- Messineo A, Ghionzoli M, Lo Piccolo R, et al. A simplified method to pass the bar through the mediastinum in the Nuss technique. Ann Thorac Surg 2015;99:717-8. [Crossref] [PubMed]
- Facchini F, Ghionzoli M, Martin A, et al. Regenerative Surgery in the Treatment of Cosmetic Defect Following Nuss Procedure. J Laparoendosc Adv Surg Tech A. 2017;27:748-53. [Crossref] [PubMed]
- Ghionzoli M, Ciuti G, Ricotti L, et al. Is a shorter bar an effective solution to avoid bar dislocation in a Nuss procedure? Ann Thorac Surg 2014;97:1022-7. [Crossref] [PubMed]
- Hebra A, Kelly RE, Ferro MM, et al. Life-threatening complications and mortality of minimally invasive pectus surgery. J Pediatr Surg 2018;53:728-32. [Crossref] [PubMed]
- Palmer B, Yedlin S, Kim S, et al. Decreased risk of complications with bilateral thoracoscopy and left-to-right mediastinal dissection during minimally invasive repair of pectus excavatum. Eur J Pediatr Surg 2007;17:81-3. [Crossref] [PubMed]
- Croitoru DP, Kelly RE Jr, Goretsky MJ, et al. The minimally invasive Nuss technique for recurrent or failed pectus excavatum repair in 50 patients. J Pediatr Surg 2005;40:181-6; discussion 186-7. [Crossref] [PubMed]
- Shin S, Goretsky MJ, Kelly RE Jr, et al. Infectious complications after the Nuss repair in a series of 863 patients. J Pediatr Surg 2007;42:87-92. [Crossref] [PubMed]
- Calkins CM, Shew S, Sharp R, et al. Management of postoperative infections after the minimally invasive pectus repair. J Pediatr Surg 2005;40:1004-7; discussion 1007-8. [Crossref] [PubMed]
- Watanabe A, Watanabe T, Obama T, et al. The use of a lateral stabilizer increases the incidence of wound trouble following the procedure. Ann Thorac Surg 2004;77:296-300. [Crossref] [PubMed]
- Krasopoulos G, Goldstraw P. Minimally invasive repair of pectus excavatum deformity. Eur J Cardiothorac Surg 2011;39:149-58. [Crossref] [PubMed]
- Lawson ML, Cash T, Akers R, et al. A pilot study of the impact of surgical repair on disease-specific quality of life among patients with pectus excavatum. J Pediatr Surg 2003;38:916-8. [Crossref] [PubMed]
- Saitoh C, Yamada A, Kosaka K, et al. Allergy to pectus bar for funnel chest. Plast Reconstr Surg 2002;110:719-21. [Crossref] [PubMed]
- Croitoru DP, Kelly RE, Goretsky MJ, et al. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Surg 2002;37:437-45. [Crossref] [PubMed]
- Haller JA Jr, Loughlin GM. Cardiorespiratory function is significantly improved following corrective surgery for severe pectus excavatum. Proposed treatment guidelines. J Cardiovasc Surg (Torino) 2000;41:125-30. [PubMed]
- Neviere R, Montaigne D, Benhamed L, et al. Cardiopulmonary response following surgical repair of pectus excavatum in adult patients. Eur J Cardiothorac Surg 2011;40:e77-82. [PubMed]
- Lawson ML, Mellins R, Tabangin M, et al. Impact of pectus excavatum on pulmonary function before and after repair with the Nuss procedure. J Pediatr Surg 2005;40:174-80. [Crossref] [PubMed]
- Coln E, Carrasco J, Coln D. Demonstrating relief of cardiac compression with the Nuss minimally invasive repair for pectus excavatum. J Pediatr Surg 2006;41:683-6. [Crossref] [PubMed]
- Malek MH, Berger DE, Housh TJ, et al. Cardiovascular function following surgical repair of pectus excavatum: a meta-analysis. Chest 2006;130:506-16. [Crossref] [PubMed]
- Kinuya K, Ueno T, Kobayashi T, et al. Tc-99m MAA SPECT in pectus excavatum: assessment of perfusion in volume changes after correction by the Nuss procedure. Clin Nucl Med 2005;30:779-82. [Crossref] [PubMed]
- Sigalet DL, Montgomery M, Harder J, et al. Long term cardiopulmonary effects of closed repair of pectus excavatum. Pediatr Surg Int 2007;23:493-7. [Crossref] [PubMed]
- Heitzer TA, Wollschlager H. Pectus excavatum with interior ischemia in right lateral position. Circulation 1998;98:605-6. [Crossref] [PubMed]
- Kelly RE Jr, Shamberger RC, Mellins RB, et al. Prospective multicenter study of surgical correction of pectus excavatum: design, perioperative complications, pain, and baseline pulmonary function facilitated by internet-based data collection. J Am Coll Surg 2007;205:205-16. [Crossref] [PubMed]
- Metzelder ML, Kuebler J, Leonhardt J, et al. Self and parental assessment after minimally invasive repair of pectus excavatum: lasting satisfaction after bar removal. Ann Thorac Surg. 2007;83:1844-9. [Crossref] [PubMed]
- Kelly RE, Cash TF, Shamberger RC, et al. Surgical repair of pectus excavatum markedly improves body image and perceived ability for physical activity: multicenter study. Pediatrics 2008;122:1218-22. [Crossref] [PubMed]
- Lam MW, Klassen AF, Montgomery CJ, et al. Quality-of-life outcomes after surgical correction of pectus excavatum: a comparison of the Ravitch and Nuss procedures. J Pediatr Surg 2008;43:819-25. [Crossref] [PubMed]
- Krasopoulos G, Dusmet M, Ladas G, et al. Nuss procedure improves the quality of life in young male adults with pectus excavatum deformity. Eur J Cardiothorac Surg 2006;29:1-5. [Crossref] [PubMed]
Cite this article as: Farronato A, Ghionzoli M, Messineo A, Politi L, Divisi D, Gonfiotti A, Crisci R. Pectus excavatum in adolescents and children: the Nuss technique. Pediatr Med 2019;2:32.