
Features and management of asynchronies in children
Introduction: asynchronies in children
Mechanical ventilation (MV) is a common and lifesaving intervention in the pediatric intensive care unit (PICU) (1,2). Unfortunately, several complications, such as ventilator-associated pneumonia, ventilator-induced lung injury (VILI) and ventilation-induced diaphragm dysfunction (VIDD) (3,4) may happen consequently.
Partially-assisted MV is the preferred mode to wean critically ill patients (4). This strategy, partitioning the ventilation between the patient and ventilator, can reduce the occurrence of VIDD by maintaining the contractile activity of the respiratory muscles (5). However, a correct optimization of the patient-ventilator interactions is mandatory to avoid both VILI and VIDD (6). Asynchrony is characterized by a mismatch between the patient and ventilator in terms of breath delivery timing. Asynchronies can be caused by several factors affecting both the patient and the ventilator (e.g., the level of sedation, the patient’s respiratory drive, the disease state and the ventilator settings) (7). The detection of asynchronies requires at least a careful clinical examination of the patients’ respiratory pattern as well as of the airway pressure (Paw) and of the flow waveforms recorded on the ventilator monitor (8). However, there are several limitations which could reduce the proper detection of asynchronies: first, asynchronies can happen anytime and it is not possible, with the current technology, to detect them continuously; second, physicians require a specific training to detect them; third, some types of asynchronies, such as auto-triggering, cannot easily be recognized by the examination of the pressure, flow, or volume waveforms.
The esophageal manometry, mainly used in the adult critical care unit to measure the esophageal pressure as a surrogate of the pleural pressure, may provide the most accurate assessment of the patient-ventilator interactions in combination with the flow, pressure and volume waveforms (9,10). However, this technique is not routinely used in the adult critical care for this scope and it is still limited for the pediatric patients (11).
If asynchronies are not properly managed, they can cause an increase the risk of VILI, VIDD, and consequently the need to increase sedation. This could impact the duration of MV and of all its complications (12).
In this review, we will classify and define the different types of asynchronies and their potential management.
Asynchronies: definitions and classification
Asynchronies are usually classified according to the phase of the respiratory cycle in which they occur: the trigger phase, the inspiration, during cycling off and the expiration phase (7). The use of this classification supports an easier diagnosis using the available waveforms (9).
However, in order to start a treatment strategy, an approach based also on the condition causing asynchronies is useful.
The respiratory drive during MV is complicated and involves feedback signals from central and peripheral chemoreceptors such as mechanoreceptors and vagal inputs from the lung, the chest wall, and the respiratory muscles (13). Respiratory centers can be also influenced by other stimuli such as anxiety and pain.
A high respiratory drive can be due to an increased metabolic demand or to compromised gas exchanges and/or intense mechanical stimuli from the lung receptors (14). A low respiratory drive, instead, can be due either to a depressed central nervous system (sedation) or to a high ventilatory support (13). Pediatric asynchronies can occur both in presence of a low respiratory drive (often related to an over-assistance) and a high respiratory drive (often related to an inadequate support) (15).
Low respiratory drive (over-assistance)
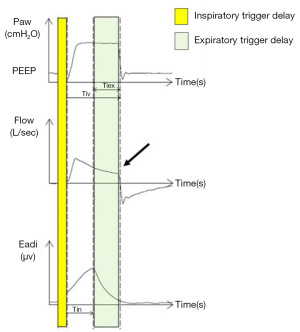
Trigger delay, ineffective triggering and delayed cycling
In children, the normal response time of both pressure or flow trigger is between 0 and 117 ms. A response time between 118 and 234 ms can defined as a trigger delay. A response time greater than 240 ms, is considered an ineffective trigger (12).
An ineffective trigger is however defined, both in children and in adults, as a sudden airway pressure drop (0.5 cmH2O) coinciding with a flow reduction, not followed by an assisted cycle during the expiratory period (16) (Figure 1). Delayed cycling occurs when the mechanical inspiratory breath continues after the end of the neural inspiration (Figure 2).
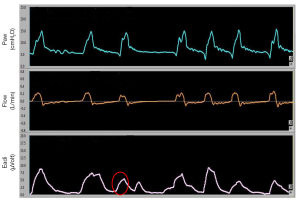
Both in adults and children, ineffective efforts are the most frequent types of asynchronies (16-20). The most frequent cause of trigger delay and ineffective efforts is the dynamic hyperinflation which is very common in pediatric obstructive pulmonary diseases (e.g., bronchiolitis, bronchodysplasia, etc.). Dynamic hyperinflation develops the “intrinsic positive end-expiratory pressure” (PEEPi), that represents an increase in the elastic load of the lungs that the patient must overcome in order to trigger the ventilator. The presence of PEEPi is determined by the presence at end expiration of a lung volume greater than the volume of air present at functional residual capacity (19-21).
Excessive trigger delay and ineffective efforts (22-25) can be corrected by implementing strategies able to reduce the dynamic hyperinflation (e.g., tidal volume reduction, reduction of the respiratory rate and of the expiratory time, and the use of an external PEEP able to counterbalance the PEEPi).
Autotrigger
The second most common type of ineffective effort is the autotriggering, that is considered in case of breaths delivered by the ventilator without an inspiratory effort (Figure 3).
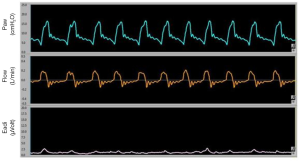
Autotriggering is characterised by an increase in volume, flow and pressure without any signs of the patient’s effort (negative deflection of the pressure waveform) (8). Mechanical causes responsible for the autotrigger are: the presence of water in the ventilator’s circuit, the presence of air leaks and the use of a low inspiratory trigger threshold. There are also patient-related causes such as a low respiratory drive or a reduced respiratory rate. In these conditions, in fact, the expiratory flow remains at zero for a long time favoring the triggering by factors other than the patient's effort. The electrical activity of the diaphragm (EAdi) can help to detect this asynchrony. Autotrigger is generally in association with a low respiratory rate and a low EAdi (<5 microVolt) (13,14). This asynchrony can be reduced by increasing the pressure or the flow threshold to trigger the ventilator, by reducing the air leaks in the circuit or by increasing the patient's respiratory drive (e.g., reducing the level of sedation, or correcting the causes of respiratory alkalosis).
Reverse triggering
Akoumianaki et al. (26) first described the occurrence of reverse triggering in adults undergoing MV. The peculiar feature of this asynchrony is that the breath is triggered by the ventilator (not by patient). Heavy sedation suppresses the respiratory drive and seems to be associated with this asynchrony.
Blokpoel et al. (27) were the first to report a reverse triggering in an11-month old infant undergoing passive MV for respiratory failure. However, more studies are needed to understand the physiologic mechanisms and the clinical impact of this asynchrony in both adult and pediatric population.
High respiratory drive (inadequate assistance)
Double triggering
Under ideal conditions, the end of the patient’s inspiration should coincide with the opening of the expiratory valve of the ventilator in order to have a passive expiration. During passive ventilation the inspiratory and expiratory phase are fixed, while during assisted modes, the end of the inspiration is recognized by a ventilator algorithm. In pressure support ventilation (PSV), the cycling off takes place when the peak inspiratory flow is reduced to a threshold value (e.g., 25% of the peak inspiratory). However, the neural inspiration may be shorter or longer than the time used by the ventilator to deliver a given flow (28,29). When the flow delivered by the ventilator stops before the end of the patient’s neural inspiration, a “double triggering” can occur (two breaths delivered by the ventilator after a single inspiratory effort) (Figure 4). Double triggering occurs in presence of two consecutive ventilator breaths separated by a short expiratory time (half of the inspiratory time or less) (16).
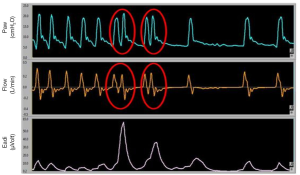
This asynchrony is easily identifiable, since the asynchronous breath is shorter than the breath that precedes it; the final lung volume can significantly increase contributing to VILI (30).
MV in pediatrics
It could be questioned whether the modern ICU ventilators are able to properly detect the inspiratory efforts in small children since their high respiratory drive.
Marchese et al. (31) tried to address this question comparing the performances of both adult and neonatal intensive care ventilator ventilators. They showed that the presence of air leaks did not affected the trigger sensitivity. In contrast, Vignaux et al. (32) demonstrated a decrease of the ventilator performances when the air leaks were introduced.
The “ideal” pediatric trigger, should have a rapid response time to cope with the short inspiratory times and the high respiratory rates and should be very sensitive to be activated by modest efforts. On the same time, it should avoid: auto-triggering, dead space effect and must compensate for air leaks. Synchronization of the MV time with the neural time is very complex especially in neonates since their short inspiratory times. Low tidal volumes, high respiratory rates, periodic breathing patterns and air leaks also contribute to the mismatch between the neural inspiratory time and the mechanical inspiratory time (32-34). These factors are challenging especially with the breath triggering, cycling-off and the breath termination.
Assisted modes of ventilation
Partial support modes allow to the contraction of the diaphragm and could contribute to reduce the occurrence of VIDD (35,36).
The aim of assisted-spontaneous breathing is to provide assisted-breaths that are synchronous to the patient’s efforts and proportionate to the patient’s requests (37,38). The actual neonatal ventilators have generally two modes of ventilation: assist-control/pressure-control ventilation (ACV/PCV) and pressure-support ventilation (PSV). The initiation of the breath can be triggered by the patient’s respiratory effort in both modes; cycling instead is controlled either by time (for PCV) or flow (for PSV), while the support level is fixed by the physician.
Proportional assisted modes of ventilation
Proportional assisted ventilation such as: neurally adjusted ventilatory assist (NAVA) and proportional-assist ventilation (PAV), could decrease the patient-ventilator asynchronies (39) while applying airways pressures proportionate to the patients’ effort (40).
During NAVA, the triggering is associated to the EAdi signal. The EAdi is a diaphragmatic electromyography recorded through an array of electrode pairs mounted on a nasogastric feeding tube (14). The EAdi is proportional to the intensity of the electrical neural stimuli to the diaphragm, i.e., to the neuro-ventilatory drive (15). When using NAVA, clinicians must set on the ventilator: (I) the FiO2; (II) the positive end expiratory pressure (PEEP); and (III) the assist level (or NAVA level), which determines the proportionality between the EAdi and the ventilator pressure in cmH2O. Cycling from inspiration to expiration occurs when the EAdi signal reaches 70% of the peak of the EAdi.
In PAV the delivered breath instead increases proportionally to the instantaneous tidal volume and inspiratory airflow generated by the patient. During PAV, the muscular effort is calculated noninvasively by the ventilator, using respiratory system elastance, flow, Paw, and resistance. Thus, the ventilator can calculate the pressure needed to support the patient accordingly to the flow and volume requested, based on the set PAV percentage. Additionally, as the patient’s lung mechanics change, those ventilators that automatically measure elastance and resistance, can adjust the amount of pressure needed to maintain the set PAV percentage. PAV is able to improve patient-ventilator interactions during the onset of inspiration, by matching the patient’s inspiratory demand. Differently in PSV, the flow decelerates when the pressure meets the targeted level.
Schulze et al. (41) randomly compared PAV vs. assisted controlled ventilation (ACV) and intermittent mandatory ventilation (IMV) in a series of 36 infants with birth weight between 600 and 1,200 g. PAV was able to maintain similar gas exchange with lower airway pressure (15% to 44% reduction depending on the index variable) (P<0.05). Furthermore, the oxygenation index decreased from 2.1 (1.7, 3.3) in ACV and 2.3 (1.7, 3.1) during IMV to 1.8 (1.3, 2.3) in PAV (P<0.05).
Musante et al. (42) also showed that PAV was able to decrease the thoraco-abdominal asynchronies and chest-wall distortion compared to continuous positive pressure airways ventilation (CPAP) in 10 preterm infants. This was permitted by an increase of 3.8–7.6 cmH2O above the CPAP level. Despite these positive studies, PAV is currently not recommended for patients less than 20 kg.
NAVA technology uses a special nasogastric tube with a series of electrodes near its distal end. The catheter must be positioned across the diaphragm to detect the electrical activity of the diaphragm during the contraction. Its use improves the patient-ventilator interactions in both adult and children (43). Similar to PAV, the ventilatory effort is proportionally shared between the ventilator and the patient. Contrary to PAV, NAVA greatly improves the inspiratory trigger, since ventilator delivers the breath when the diaphragm is “electrically” stimulated. The EAdi monitoring in neonatal and pediatric patients seemed to improve the detection of patient-ventilator asynchronies in patients ventilated not only in NAVA, but also in other ventilatory modes (44-48). Beck et al. (47) reported improved patient-ventilator interactions in 7 low-birth-weight neonates even in presence of large air leaks. Neonates on conventional ventilation had a similar mechanical and neural inspiratory time while the cycling off in conventional ventilation was 120 milliseconds earlier than in NAVA. They also found the neural expiratory times and respiratory rates were shorter during NAVA. Bengtsson et al. (48) found improved patient-ventilator interactions during a 4-hour trial in NAVA compared with PSV in 16 children. They also found a reduction of 28% of the peak airway pressure at the beginning of NAVA and a reduction of the 32% of the peak airway pressure after 3 hours of NAVA. Mean airway pressure, minute ventilation, expired tidal volume, respiratory rate, heart rate, PaO2 and PaCO2 did not change. This study did not show any patients or device-adverse events. Bordessoule et al. (49) evaluated 10 infants in NAVA, pressure control ventilation (PCV) and PSV. In PCV and PSV, 4% and 6.5%, respectively, of the neural efforts did not trigger the ventilator. In NAVA, instead, all the inspiratory efforts were triggered. Trigger inspiratory delay was higher in PCV (193 milliseconds) and PSV (135 milliseconds) than in NAVA (93 milliseconds) and the ventilator cycled off before the end of neural inspiration both in PCV and PSC (in 12% and in 21% of the breaths respectively).
Conclusions
Asynchronies are frequent events during MV. Different types of asynchronies arise from different physiologic causes, thus management changes accordingly. Our knowledge about pediatric asynchronies derives from small and limited observational studies, therefore precise epidemiologic data are needed. Future pediatric studies should evaluate the real benefit of a continuous monitoring of asynchronies at bedside (esophageal catheter or EAdi) to increase their detection and evaluate if this approach can impact the final outcome (reduction of MVs free days and survival).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pm.2019.08.05). MDN serves as an unpaid editorial board member of Pediatric Medicine from Jul 2018 to Jun 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Briassoulis G, Filippou O, Natsi L, et al. Acute and chronic paediatric intensive care patients: current trends and perspectives on resource utilization. QJM 2004;97:507-18. [Crossref] [PubMed]
- Martinot A, Leteurtre S, Grandbastien B, et al. Characteristics of patients and use of resource in French pediatric intensive care units. Le groupe francophone de Rèanimation et urgences pédiatriques. Arch Pediatr 1997;4:730-6. [Crossref] [PubMed]
- Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med 2004;169:336-41. [Crossref] [PubMed]
- Goligher EC, Fan E, Herridge MS, et al. Evolution of Diaphragm Thickness during Mechanical Ventilation. Impact of Inspiratory Effort. Am J Respir Crit Care Med 2015;192:1080-8. [Crossref] [PubMed]
- Martin AD, Joseph AM, Beaver TM, et al. Effect of intermittent phrenic nerve stimulation during cardiothoracic surgery on mitochondrial respiration in the human diaphragm. Crit Care Med 2014;42:e152-6. [Crossref] [PubMed]
- Goligher EC, Brochard LJ, Reid WD, et al. Diaphragmatic myotrauma: a mediator of prolonged ventilation and poor patient outcomes in acute respiratory failure. Lancet Respir Med 2019;7:90-8. [Crossref] [PubMed]
- Kondili E, Prinianakis G, Georgopoulos D. Patient-ventilator interaction. Br J Anaesth 2003;91:106-19. [Crossref] [PubMed]
- Georgopoulos D, Prinianakis G, Kondili E. Bedside waveforms interpretation as a tool to identify patient-ventilator asynchronies. Intensive Care Med 2006;32:34-47. [Crossref] [PubMed]
- Colombo D, Cammarota G, Alemani M, et al. Efficacy of ventilator waveforms observation in detecting patient-ventilator asynchrony. Crit Care Med 2011;39:2452-7. [Crossref] [PubMed]
- de Wit M. Monitoring of patient-ventilator interaction at the bedside. Respir Care 2011;56:61-72. [Crossref] [PubMed]
- Cheifetz IM. Pediatric ARDS. Respir Care 2017;62:718-31. [Crossref] [PubMed]
- Blokpoel RG, Burgerhof JG, Markhorst DG, et al. Patient-Ventilator Asynchrony During Assisted Ventilation in Children. Pediatr Crit Care Med 2016;17:e204-11. [Crossref] [PubMed]
- Georgopoulos D, Roussos C. Control of breathing in mechanically ventilated patients. Eur Respir J 1996;9:2151-60. [Crossref] [PubMed]
- Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med 2017;195:438-42. [Crossref] [PubMed]
- Pham T, Telias I, Piraino T, et al. Asynchrony Consequences and Management. Crit Care Clin 2018;34:325-41. [Crossref] [PubMed]
- Thille AW, Rodriguez P, Cabello B, et al. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 2006;32:1515-22. [Crossref] [PubMed]
- Piquilloud L, Vignaux L, Bialais E, et al. Neurally adjusted ventilatory assist improves patient-ventilator interaction. Intensive Care Med 2011;37:263-71. [Crossref] [PubMed]
- Chao DC, Scheinhorn DJ, Stearn-Hassenpflug M. Patient-ventilator trigger asynchrony in prolonged mechanical ventilation. Chest 1997;112:1592-9. [Crossref] [PubMed]
- de Wit M, Pedram S, Best AM, et al. Observational study of patient-ventilator asynchrony and relationship to sedation level. J Crit Care 2009;24:74-80. [Crossref] [PubMed]
- Blanch L, Villagra A, Sales B, et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med 2015;41:633-41. [Crossref] [PubMed]
- Beck J, Gottfried SB, Navalesi P, et al. Electrical activity of the diaphragm during pressure support ventilation in acute respiratory failure. Am J Respir Crit Care Med 2001;164:419-24. [Crossref] [PubMed]
- Nava S, Bruschi C, Rubini F, et al. Respiratory response and inspiratory effort during pressure support ventilation in COPD patients. Intensive Care Med 1995;21:871-9. [Crossref] [PubMed]
- Smith TC, Marini JJ. Impact of PEEP on lung mechanics and work of breathing in severe airflow obstruction. J Appl Physiol (1985) 1988;65:1488-99. [PubMed]
- Mancebo J, Albaladejo P, Touchard D, et al. Airway occlusion pressure to titrate positive end-expiratory pressure in patients with dynamic hyperinflation. Anesthesiology 2000;93:81-90. [Crossref] [PubMed]
- MacIntyre NR, Cheng KC, McConnell R. Applied PEEP during pressure support reduces the inspiratory threshold load of intrinsic PEEP. Chest 1997;111:188-93. [Crossref] [PubMed]
- Akoumianaki E, Lyazidi A, Rey N, et al. Mechanical ventilation-induced reverse-triggered breaths: a frequently unrecognized form of neuromechanical coupling. Chest 2013;143:927-38. [Crossref] [PubMed]
- Blokpoel RGT, Wolthuis DW, Koopman AA, et al. Reverse Triggering: A Novel Type of Patient-Ventilator Asynchrony in Mechanically Ventilated Children. Am J Respir Crit Care Med 2019;200:e4-e5. [Crossref] [PubMed]
- Ranieri VM, Giuliani R, Mascia L, et al. Patient-ventilator interaction during acute hypercapnia: pressure-support vs. proportional-assist ventilation. J Appl Physiol (1985) 1996;81:426-36. [PubMed]
- Yamada Y, Du HL. Analysis of the mechanisms of expiratory asynchrony in pressure support ventilation: a mathematical approach. J Appl Physiol (1985) 2000;88:2143-50. [PubMed]
- Yoshida T, Fujino Y, Amato MB, et al. Fifty Years of Research in ARDS. Spontaneous Breathing during Mechanical Ventilation. Risks, Mechanisms, and Management. Am J Respir Crit Care Med 2017;195:985-92. [Crossref] [PubMed]
- Marchese AD, Chipman D, de la Oliva P, et al. Adult ICU ventilators to provide neonatal ventilation: a lung simulator study. Intensive Care Med 2009;35:631-8. [Crossref] [PubMed]
- Vignaux L, Piquilloud L, Tourneux P, et al. Neonatal and adult ICU ventilators to provide ventilation in neonates, infants, and children: a bench model study. Respir Care 2014;59:1463-75. [Crossref] [PubMed]
- Keszler M. State of the art in conventional mechanical ventilation. J Perinatol 2009;29:262-75. [Crossref] [PubMed]
- Bhandari V. Synchronized ventilation in neonates: a brief review. Neonatology Today 2011;6:1-6.
- Petrof BJ, Jaber S, Matecki S. Ventilator-induced diaphragmatic dysfunction. Curr Opin Crit Care 2010;16:19-25. [Crossref] [PubMed]
- Slutsky AS. Lung injury caused by mechanical ventilation. Chest 1999;116:9S-15S. [Crossref] [PubMed]
- Sinderby C, Beck J. Neurally adjusted ventilatory assist (NAVA): An update and summary of experiences. Neth J Crit Care 2007;11:243-52.
- Beck J, Campoccia F, Allo JC, et al. Improved synchrony and respiratory unloading by neurally adjusted ventilatory assist (NAVA) in lung-injured rabbits. Pediatr Res 2007;61:289-94. [Crossref] [PubMed]
- Schmidt M, Kindler F, Cecchini J, et al. Neurally adjusted ventilatory assist and proportional assist ventilation both improve patient-ventilator interaction. Crit Care 2015;19:56. [Crossref] [PubMed]
- Lellouche F, Brochard L. Advanced closed loops during mechanical ventilation (PAV, NAVA, ASV, SmartCare). Best Pract Res Clin Anaesthesiol 2009;23:81-93. [Crossref] [PubMed]
- Schulze A, Gerhardt T, Musante G, et al. Proportional assist ventilation in low birth weight infants with acute respiratory disease: A comparison to assist/control and conventional mechanical ventilation. J Pediatr 1999;135:339-44. [Crossref] [PubMed]
- Musante G, Schulze A, Gerhardt T, et al. Proportional assist ventilation decreases thoracoabdominal asynchrony and chest wall distortion in preterm infants. Pediatr Res 2001;49:175-80. [Crossref] [PubMed]
- Sinderby C, Beck J. Proportional assist ventilation and neurally adjusted ventilatory assist--better approaches to patient ventilator synchrony?. Clin Chest Med 2008;29:329-42. vii. [Crossref] [PubMed]
- Breatnach C, Conlon NP, Stack M, et al. A prospective crossover comparison of neurally adjusted ventilatory assist and pressure-support ventilation in a pediatric and neonatal intensive care unit population. Pediatr Crit Care Med 2010;11:7-11. [Crossref] [PubMed]
- Alander M, Peltoniemi O, Pokka T, et al. Comparison of pressure-, flow-, and NAVA-triggering in pediatric and neonatal ventilatory care. Pediatr Pulmonol 2012;47:76-83. [Crossref] [PubMed]
- Clement KC, Thurman TL, Holt SJ, et al. Neurally triggered breaths reduce trigger delay and improve ventilator response times in ventilated infants with bronchiolitis. Intensive Care Med 2011;37:1826-32. [Crossref] [PubMed]
- Beck J, Reilly M, Grasselli G, et al. Patient-ventilator interaction during neurally adjusted ventilatory assist in low birth weight infants. Pediatr Res 2009;65:663-8. [Crossref] [PubMed]
- Bengtsson JA, Edberg KE. Neurally adjusted ventilatory assist in children: an observational study. Pediatr Crit Care Med 2010;11:253-7. [Crossref] [PubMed]
- Bordessoule A, Emeriaud G, Morneau S, et al. Neurally adjusted ventilatory assist improves patient–ventilator interaction in infants as compared with conventional ventilation. Pediatr Res 2012;72:194-202. [Crossref] [PubMed]
Cite this article as: Lonero M, Di Nardo M. Features and management of asynchronies in children. Pediatr Med 2019;2:50.

